Keywords
|
| High performance liquid chromatography (HPLC), Imidacloprid Residue, Goat, Tissues. |
Introduction
|
| Imidacloprid belongs to a new chemical class of active ingredient. It has a new mode of action, outstanding biological efficacy, a broad spectrum of activities, low toxicity to warmblooded animals [1]. Imidacloprid is a systemic insecticide, chemically belonging to the neonicotinoid group and acts on the nervous system. It acts by disrupting nicotinic acetylcholine receptors in the central nervous system [2]. The selective toxicity of imidacloprid to insects and not to mammals is attributed to differences in the binding affinity or potency at nicotinic acetylcholine receptors [3]. Since being introduced in the insecticide market in 1992, the use of imidacloprid has increased over the years. It is a category II acute toxicant and thus, is classified as a General Use Pesticide [4]. In veterinary medicine, it is used as a flea control agent on dogs and cats [5]. Indiscriminate usage of pesticides in agriculture is leading to contamination of the environment and natural resources. It is being greatly criticized due to their persistence in the environment and their accumulation in the living tissues of organisms and thereby, producing an adverse impact on animal and human health [6]. Repeated exposure to the insecticide may leave some residue in tissues. Therefore, it is essential to validate a method for estimation of residues of imidacloprid in animal tissues. The literature on determination of imidacloprid residues in plants and vegetables are many but regarding residue estimation of imidacloprid in animal is scarcely available. Therefore, the present work was undertaken to find out an easy and simple method for determination of imidacloprid residues in animal tissues. The method consists of reversed phase by high performance liquid chromatography with photo diode array (PDA) detector. |
Materials and Methods
|
| Analytical reference standard of imidacloprid was obtained from M/s United Phosphorus Limited Mumbai, India. The purity of imidacloprid was checked to be of ≥ 98.5% purity. All other chemicals and solvents used in the study was analytical and HPLC grade. |
Collection of samples
|
| Three adult goats weighing 10-12 kg were used for the experiment. Three samples of each tissue viz. blood, skeletal muscle, liver and kidney were collected from three animals. Blood samples (5 ml each) were collected from the left jugular vein in heparinized test tubes; whereas 1 gm of tissue from skeletal muscle, liver, kidney were collected following slaughter of animals. |
| This study was approved by the Institutional Animal Ethics Committee, Faculty of Veterinary and Animal Sciences, West Bengal University of Animal and Fishery Sciences, Kolkata, India. |
Fortification of samples
|
| Three numbers of samples of each tissue from three different animals was collected for analysis of imidacloprid. Recovery percentage of imidacloprid from different tissues was carried out to ascertain the reliability of the analytical method after fortifying the different tissues namely, blood plasma, liver, kidney and skeletal muscle with technical grade imidacloprid of different spike concentration e.g., 0.01, 0.1, 1.0, 5.0, 10.0, 12.5 μg/ml. After necessary work up, the concentrations of imidacloprid from different tissues was analyzed by high performance liquid chromatography (HPLC) [7-8] equipped with PDA detector. |
Extraction: Blood:
|
| Blood samples (5 ml each) were collected from the left jugular vein in heparinized test tubes. Plasma was separated by centrifugation at 3000 rpm for 20 minutes. The blood samples were subjected to liquid-phase extraction [9]. To a sample of plasma (1 ml), acetonitrile (5 ml) was added, shaken for 5 min, filtered through filter paper no. 1. This process was repeated thrice. The total filtrate was evaporated to dryness using rotary vacuum evaporator. The final volume was made up by HPLC grade acetonitrile (1 ml) for subsequent HPLC analysis (Scheme I) [10]. |
| Tissues: Liver, kidney, skeletal muscle. |
| The tissue (1 gm) was first minced with scissors and then homogenized in Remi-tissue homogenizer (RQ-127A) for 4 minutes with acetonitrile (5 ml). The homogenate was filtered through filter paper (Whatman No. 1) and the remnants of tissue were re-homogenised and re-extracted twice with acetonitrile (5 × 2 ml) and filtered through above filter paper (Scheme II) [10]. |
Clean up
|
| The acetonitrile filtrates were transferred to a clean, dry separatory funnel and n-hexane was added to it (1:1). The mixture was shaken vigorously for 2 min and allowed to settle (2 min) till the two phases were distinctly separated. The lower acetonitrile phase was collected in another separatory funnel and re-partitioned twice with n-hexane. The acetonitrile phase was then collected in another conical flask and evaporated to dryness using rotary vacuum evaporator. Final volume was made to 1 ml with acetonitrile (HPLC grade). The sample was filtered through 0.20μ membrane filter before injection to HPLC (Scheme II) [10]. |
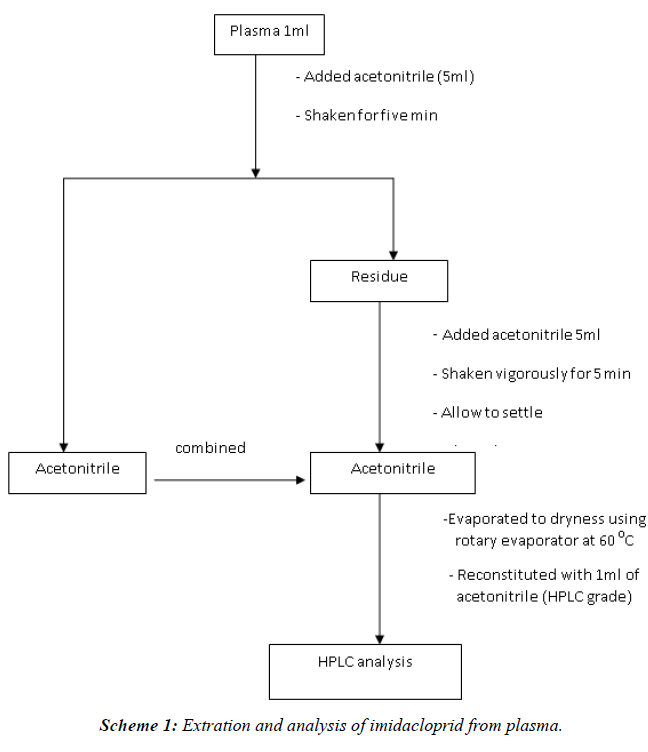 |
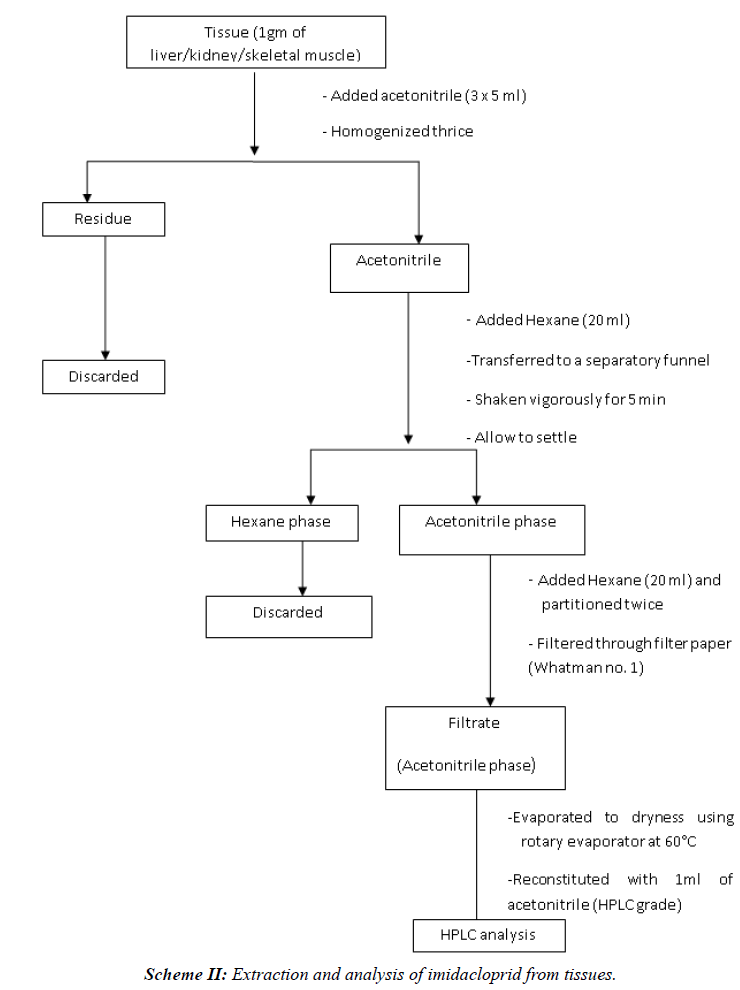 |
Estimation of Concentration of insecticide
|
| Apparatus: SHIMADZU LC-20 AT liquid chromatogram coupled with Photo Diode-Array detector attached with computer SPD-MXA 10 software. |
HPLC condition for Imidacloprid
|
| • Mobile phase: Acetonitrile and Water (90:10). This mixture was subjected to membrane filtration. |
| • Column : 5μ Luna C18 (2); 250 × 4.6 mm (RP) |
| • Flow rate : 1 ml/min |
| • Wave length : 270 nm |
| • Retention time: 3.30 min |
Estimation of Imidacloprid
|
| Standard and sample (20 μl each) were injected by Hamilton Syringe into the injector port of liquid chromatography with the first and last being the standard. The residues were estimated after comparing with external standard. |
| The concentration of Imidacloprid in blood and tissue was calculated using the following equation: |
| Concentration of Imidacloprid in blood (μg/ml) = a2 × v2 × C/ a1× v1 |
| Residue of Imidacloprid in tissues (μg/gm) = a2 × v2 × C/ a1× g |
| a1=area of standard chromatogram |
| a2=area of sample chromatogram |
| V2=Final volume of sample after processing (ml) |
| V1=Volume of plasma and urine taken for processing (ml) |
| C=concentration of standard |
| g=amount of tissue taken for processing |
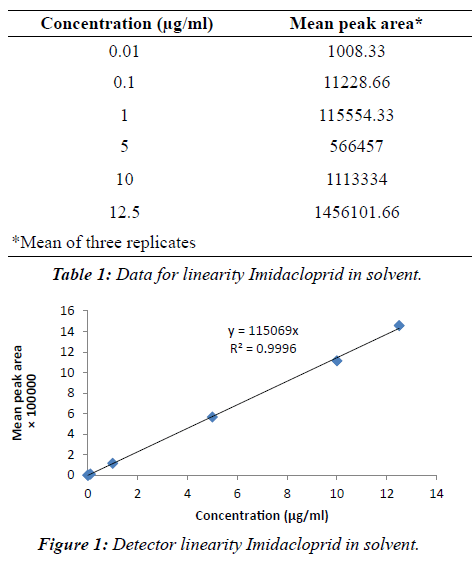 |
Results and Discussion
|
| The linearity of the detector response was tested for imidacloprid in solvent over the range of 0.01 μg/mlto 12.50 μg/ml (Table 1). A very precise linear relation between the injected amount and the resulting peak area was observed over the entire range with correlation coefficient between 0.9958 and 0.9996 (Figure 1). Imidacloprid estimated by reverse phase high performance liquid chromatography (RP-HPLC). The chromatogram (Figures 2 and 3) showed a well resolved peak of imidacloprid and the retention time was at 3.30 minutes under the operating condition of the chromatogram. The method is satisfactory and subsequently used for extraction, clean up and estimation of imidacloprid from blood, and other tissues of goat (Schemes I and II). A summary of the obtained recovery values is given in Table 2. The repeatability of the method was determined by running a set of three recoveries. The resulting mean recovery rates ranged from 93 to 97% with relative standard deviations (RSD) between 0.52% and 0.88% (Table 2). Limit of detection (LOD) was 0.01 μg/ ml and limit of quantification (LOQ) was found to be 0.04 μg/ml. These data demonstrate the excellent sensitivity, selectivity and precision of the method. Therefore, this method may be adapted to determine the imidacloprid residue level in animal tissues. The study reveals that the extraction of samples with acetonitrile and hexane are suitable for imidacloprid analysis. The separation and quantification of imidacloprid by RP-HPLC is better at wavelength of 270 nm with mobile phase of acetonitrile and water (90:10 v/v). The method is most suitable for rapid precise analysis of imidacloprid in animal tissues. |
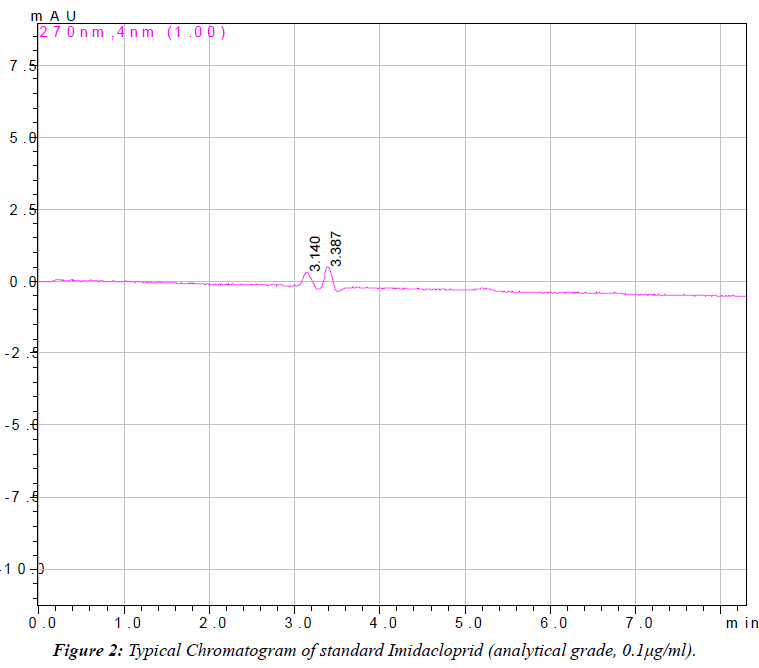 |
 |
|
|
References
- 1. Schoning R, Schmuck R. Analytical determination and relevant metabolite residues by LC MS/MS. Bulletin of Insectology 2003; 56: 41-50.
- 2. Fossen M. Environmental fate of imidacloprid. California Department of Pesticide Regulation. Summary of pesticide use report data indexed by chemical.
- 3. Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: Mechanisms of Selective Action. Annual Review of Pharmacology and Toxicology 2004; 45: 247-268.
- 4. US Environmental protection agency. Drinking Water Infrastructure Needs Survey and Assessment Fourth Report to Congress. 2009.
- 5. Hutchinson MJ, Jacobs DE, Mencke N. Establishment of the cat flea (Ctenocephalidesfelis) on the ferret (Mustelaputoriusfuro) and its control with imidacloprid. Medical and Veterinary Entomology 2001; 15: 212-214.
- 6. Moran KD. Insecticide Market Trends and Potential Water Quality Implications, prepared for the San Francisco Bay Regional Water Quality Control Board and the San Francisco Estuary Project 2003.
- 7. Samnani K, Vishwakarma K, Pandey S. Simple and Sensitive method for Determination of Imidacloprid Residue in Soil and Water by HPLC. Bulletin of Environmental Contamination and Toxicology 2011; 86: 554- 558.
- 8. Yan HR, Xulun Z, Zhu ZZ, Mei CH, Wan X. A method for determination of imidacloprid residue in tea with HPLC-UV. Journal of Tea Science 2009; 29: 67-71.
- 9. Wilson K, Walker J (eds.). Principles and Techniques of Biochemistry and Molecular Biology, Cambridge University Press 2006.
- 10. Dewangan G, Patra PH, Mishra A, Singh AK, Dutta BK, Sar TK, Chakraborty AK, Mandal TK. Haemobiochemical, immunological, antioxidant status and residues of flumethrin following weekly dermal application in goats. Toxicological & Environmental Chemistry 2012; 94: 377-387.
|




