Keywords |
| Tenofovir disoproxil fumarate (TDF), Nephrotoxicity, Rat, Proximal tubular
dysfunction |
Introduction |
| Tenofovir disoproxil fumarate (TDF) is an oral prodrug of
tenofovir, which exhibits activity against HIV-1 reverse
transcriptase [1, 2]. It is at present the only nucleotide
analogue reverse-transcriptase inhibitor (NRTI) approved
by the US Food and Drug administration (FDA) for the
treatment of AIDS [3]. It is primarily excreted through the
kidney via glomerular filtration and active tubular secretion.
The 300-mg/day oral TDF regimen [3] is preferred to
other anti-retrovirals such as adenofir and cidofir because
of its convenience, efficacy, safety, and tolerability [4-6].
However recent studies show that TDF has serious side
effects, especially with long-term use. |
| Nephrotoxicity due to tenofovir treatment of HIV patients
has been reported over the past few years [7-11]. Among
the principal side effects associated with TDF use were
hypophosphatemia, [11-13], renal failure, [14,15] and
tubular toxicity [16]. The main site of toxicity appears to
be the proximal tubule, and in more severe cases, patients
can develop Fanconi syndrome (which is characterized by tubular proteinuria, aminoaciduria,
phosphaturia,
glycosuria, and bicarbonate wasting (leading to metabolic
acidosis) or acute kidney injury [17]. Several case reports,
observational studies, animal models and cell culture data
support that tenofovir is nephrotoxic for proximal tubular
cells [18-21]. It has been suggested that TDF can cause
direct proximal tubular damage, which may lead to renal
failure [22]. |
| It is well established that TDF targets the mitochondria
of the proximal tubular epithelium. Morphological
evidence of mitochondrial toxicity has been reported
in human biopsies of tenofovir treated HIV patients
[13,18]. In the TDF treated HIV patients who underwent
kidney biopsy, the main abnormality on light microscopy
was acute proximal tubule damage, and eosinophilic
intracytoplasmic inclusions formed of giant mitochondria
[13]. Electron microscopy showed mitochondria of marked
variations in size and shape and disruption of cristae,
mitochondrial swelling, and accumulation of crystals in
the mitochondrial matrix [18]. However, how TDF causes mitochondrial damage is not clear. In
order to investigate
TDF nephrotoxicity, a reliable animal model is necessary.
Although a few models have been proposed earlier, to date
there is no standard animal model that resembles that of
humans histologically and biochemically. In the present
study we describe a reliable and reproducible rat model
of TDF nephrotoxicity which resembles that of humans
both histologically and biochemically. This model may be
useful to study the nephrotoxicity of TDF as well as to
carry out intervention studies. |
Methods |
Animals and animal treatment |
| The rat is proven to be a useful model for the study of
nephrotoxicity of a number of agents including gentamicin,
an antibiotic, cisplatin, a chemotherapeutic drug, and
acetaminophen, an antipyretic drug. Therefore, we chose
to standardise a rat model of TDF nephrotoxicity. |
| Adult male Wistar rats (200-250 g) were used for the
studies. They were housed in standard rat cages (421 ×
290 × 190 mm). All animals were exposed to 12 hour
light-dark cycles and allowed access ad libitum to water
and rat chow. The experiments done were approved by
the institutional animal ethics committee and were in
accordance with the guidelines of the Committee for the
Purpose of Control and Supervision of Experimentation
on Animals (CPCSEA), Government of India. |
Study 1 |
| The daily dose of TDF in humans is 300 mg/60 kg [3].
Therefore to remain clinically relevant, we administered
TDF daily by gavage (morning) to adult male Wistar rats
at doses that resembled human therapy on an mg/kg/day
basis i.e., 5 mg/kg; -250 g rat=1.25 mg/day) for 5 weeks.
Based on a number of NRTI treatment protocols used by
other workers, maximum treatment duration of 5 weeks
was used in this study as the duration of treatment for 5
weeks is suggested to model chronic human treatment
[23]. The rats were sacrificed 24 hrs. After the final dose
of TDF, the kidneys processed for histological studies. |
| Glomeruli and tubules appeared to be intact in the
TDF-treated rats. EM studies showed normal renal
proximal tubular epithelial cells with characteristic, oval
mitochondria having densely packed cristae (Figure not
shown). |
Study 2 |
| We next tried the dose that was used by Lebrecht et al.
[21]. Three adult male Wistar rats were treated by oral
gavage once a day for 8 weeks with 100 mg/kg body
wt. of TDF. Based on area under the curve exposure, the
TDF dose used in these rats is about twice the clinical
dose used in patients [3]. We could not find any renal
abnormalities when examined by light microscopy and
electron microscopy. Therefore we tried a higher dose that
was used by Biesecker et al. [24]. |
Study 3 |
| Biesecker et al., [24] have tried a higher dose of TDF
-300 mg/kg body weight/day (which is 6 × the human
dose) over a period of 4 weeks. Treatment related clinical
observations reported by them were slight increase in blood
urea nitrogen (BUN) with normal creatinine, decrease in
urinary phosphorus and increase calcium. Minimal renal
proximal tubular epithelial karyomegaly was observed but
they could not find any mitochondrial abnormalities. When
we tried 300 mg/kg body weight/day for 4 weeks on 3
adult male Wistar rats we also could not find any proximal
tubular abnormalities (figure not shown). Therefore, we
next checked whether a longer duration of treatment i.e.,
300 mg/kg body wt. per day for 8 weeks produces renal
tubular toxicity. No mitochondrial injury was observed by
electron microscopy in the rats at this dose and duration of
treatment also (Figure 1). |
Study 4 |
| Tenofovir has been shown to cause bone toxicity in
animal models, when given at 6?12 times higher dose than
recommended for humans [25]. Therefore, we tried 600
mg/kg body weight/day (corresponds to 12 × human dose).
Based on a number of NRTI treatment protocols used by
other workers, maximum treatment duration of 5 weeks
was used in these studies as the duration of treatment for
5 weeks is suggested to model chronic human treatment
[29]. |
| We administered 600 mg TDF/kg body weight/day in
two divided dose of by gavage for 5 weeks to 3 adult
male Wistar rats. TDF treatment for 5 weeks showed severe morphologic abnormalities in
proximal tubule
mitochondria, such as variations in size and shape (giant
mitochondria), disruption of cristae, mitochondrial
swelling, and presence of amorphous deposits in the
mitochondrial matrix, the findings that were in close
comparison with human kidney biopsies from TDF treated
HIV patients. |
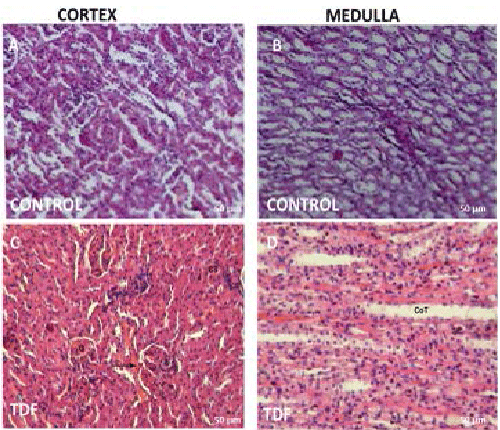 |
| Figure 1: Representative light micrographs of rat kidney. (A)
Renal cortex of a control rat showing normal architecture
[H & E, 200X]; (B) Renal medulla of a control rat showing
normal architecture [H & E, 200X]; (C) Renal cortex of
a 300 mg/kg/d TDF treated rat showing almost normal
structure, (H & E, 200X); (D) Renal medulla of a 300 mg/
kg/d TDF treated rat showing normal architecture (H & E,
200X). |
Standardisation of rat model of TDF tubulopathy |
| The rats were assigned randomly into 2 groups and were
treated as follow. |
| Group I (control): The rats in this group (n=3) received
sterile water. |
| Group II: The rats (n=6) in this group received 600 mg/
kg body weight TDF divided into two doses, one in the
morning and one in the evening by gavage for 5 weeks. |
| Control animals were treated with sterile water on the
same schedule as TDF treatment and were killed at the
same time point for TDF treated and control rats. |
Mortality check and body weights |
| Animals were checked daily for morbidity or death. Body
weights were measured daily just before gavage dosing. |
Collection of blood, urine and kidneys |
| Twenty-four hours before sacrifice, the rats were placed
individually in metabolic cages, and urine was collected
for biochemical analysis. On the 36th day, after overnight
fast, blood samples were collected from the rats under
halothane anesthesia, by cardiac puncture into tubes and
allowed to clot at room temperature. Thereafter, serum was
separated by centrifugation at 1200 g for 15 min at 4°C
for clinical chemistry. Then the animals were sacrificed
by over dose of halothane anesthesia. The abdomen was
opened by midline incision and kidney was dissected
out carefully and cleaned off the extraneous tissue and
weighed. Half of left kidney was cut in cross-section and
fixed in 10% buffered formalin for light microscopy, and
the remaining half was fixed in 3% glutaraldehyde for
electron microscopy. |
Morphological examination of the kidney |
| After fixation of kidney tissues in 10% buffered formalin
for 24 h at room temperature, the slices were embedded
in paraffin and then sectioned. Four micrometer-thick
paraffin sections were stained with hematoxylin and eosin
for light microscope examination using conventional
protocol [26]. A minimum of 8 fields for each kidney
section were examined and assigned for severity of
changes by an observer blinded to the treatments of the
animals. Since the tenofovir-induced morphological
abnormalities in rat kidney are mainly localized in the
proximal tubules, and the other structures of the kidney do
not exhibit major histological alterations, only the renal
cortex was examined in detail. |
Examination of the ultrastructural changes in the kidney
tissues by electron microscope (EM) |
| Electron microscopy was done based on the methods
employed routinely in the Lewis lab [27]. The kidney
tissues were fixed in 3% glutaraldehyde and washed in
buffer, post fixed by 1% osmium tetraoxide and washed
in buffer, and, dehydrated in increasing concentrations
of alcohol. The tissues were washed with propylene
oxide and embedded in epoxy-resin embedding medium.
Sections (0.5 µ) were cut with glass knives and stained
with Toluidine Blue for orientation. Ultrathin (900 Ã
)
sections were cut with a diamond knife, stained with uranyl
acetate and lead citrate and examined by EM, evaluated
and photographed. Each EM photomicrograph was
reviewed independently by two investigators. Parameters
included presence of structurally abnormal mitochondria,
numbers of mitochondrial profiles per field, mitochondrial
swelling, abnormal cristae density, cristae disruption, and
accumulation of intra-mitochondrial crystals [28]. |
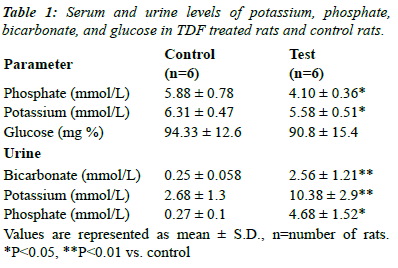
Serum clinical chemistry |
| Serum was separated out and used for the estimation of
phosphate, potassium, bicarbonate, glucose, urea and
creatinine by standard spectrophotometric methods. |
Urinalysis |
| Characteristic features associated with mitochondrial
dysfunction in proximal tubular cells include phosphaturia,
bicarbonate wasting, tubular proteinuria, glycosuria, and
aminoaciduria - acquired Fanconi Syndrome [22]. Urine
samples were centrifuged to remove suspended material,
and the supernatants were used for the estimation of
bicarbonate, phosphate, and potassium by standard
spectrophotometric methods. Glucose and protein were
semi quantified by dipstick. Low molecular weight
proteins in urine were detected by SDS PAGE. |
Detection of low molecular weight proteins in urine by
SDS PAGE |
| Urine proteins were measured by Lowry?s method and
fractionated by SDS-PAGE using 8% resolving gel and
5% stacking gel [29]. Each sample containing 100 µg of
urinary protein was mixed with protein dissociation buffer
in the ratio of 1:1 and kept in a boiling water bath for 5
mins. Samples were briefly centrifuged; they were then
loaded onto wells. Running gel buffer (pH 8.6) was added
to electrophoresis tank. The apparatus was connected to the
power pack and was run at 70V till the sample reached the
separating gel. The voltage applied was increased to 90V at this point. Electrophoresis was
stopped when the marker
dye reached near the end of the gel. After electrophoretic
separation, the gel was stained with Coomassie blue
solution (0.01% Coomassie brilliant blue R 250, 50% (v/v)
methanol and10% (v/v) glacial acetic acid) for 3 h at room
temperature and subsequently destained in the destaining
solution (50% (v/v) methanol and 10% (v/v) acetic acid)
for 2 h. The gel image was captured and analysed by a gel
documentation system (Alpha Innotech). |
Assessment of mitochondrial function using respiratory
control ratio (RCR) |
| The respiratory control ratio is the single most useful
general measure of function in isolated mitochondria.
High RCR indicates good function, and low RCR usually
indicates dysfunction. Therefor the RCR ratio was carried
out on mitochondria isolated from the kidney. |
Isolation of kidney mitochondria |
| The kidney tissues were homogenized (5%) using the
homogenizing buffer consisting of 220 mM Mannitol/70
mM sucrose/5 mM Tris/1 mM EGTA; pH 7.4. The
homogenates were centrifuged at 4000 × g for 10 min,
and the nuclear pellet was discarded. Crude mitochondrial
fractions were obtained by centrifuging at 12,000×g
for 20 min, and the pellet was washed thrice with wash
buffer containing 220 mM mannitol/70 mM sucrose/20
mM HEPES; pH 7.4 [30]. The final pellet was suspended
in the same buffer. The purity of the mitochondria was
established by enrichment of marker enzyme, succinate
dehydrogenase. The activity of succinate dehydrogenase
was assayed using INT as an electron acceptor, which
forms formazan crystals on reduction [31]. The isolated
mitochondria were used for assessing RCR. |
Measurement of oxygen uptake (RCR) |
| Oxygen uptake was determined polarographically using
a Clark-type electrode in 2 ml of respiratory buffer (225
mM sucrose, 5 mM MgCl2, 10 mM KH2PO4, 20 mM
KCl, 10 mM Tris, and 5 mM HEPES pH 7.4), containing
5 mM succinate as a respiratory substrate [32]. About 2
mg/ml of mitochondrial protein was introduced into the
oxygen electrode compartment (Rank oxygen electrode).
The electrode output was connected to an appropriate
recorder, and the recorder was set such that 100% full
range corresponded to the total oxygen content of the
mixture. Oxygen uptake was stimulated with 0.3 mM
ADP, and the rate of states 3 and 4 respirations was
measured. Oxygen uptake was calculated from the
decrease in the percentage saturation of the mixture. The ratio of state 3/state 4 respiratory
rates was calculated
for RCR. |
Statistical Analysis |
| The results are expressed as mean ± SD. Significant
statistical differences between the two groups were
evaluated using Student?s t test. P value = 0.05 was taken
as statistically significant. |
Results |
Effect of TDF administration on morbidity or death of
rats |
| All the rats (control and TDF treated) survived the
treatment period of 5 weeks. The rats did not show any
signs of morbidity. |
Chronic TDF treatment decreases the body wt. and
kidney weight of rats |
| There was significant difference in body weight and kidney
weights between control and TDF treated rats at the time
of sacrifice (Figure 2). The kidney/body weight ratio was
significantly lower in the TDF treated rats as compared
with control. |
Chronic TDF treatment results in proximal tubular
atrophy and degeneration |
| Since the tenofovir-induced morphological abnormalities
in are mainly localized in the proximal tubules , and
the other structures of the kidney do not exhibit major
histological alterations, only the renal cortex was
examined in detail. The kidneys of control rats showed
normal morphology. Sections from control group showed
normal histological structure of the glomeruli and renal
tubules in the cortex (Figure 3A) and normal tubules in the
medulla (Figure 3B). TDF induced renal damage involved
mainly the cortex and to a lesser extent the medulla. The
proximal convoluted tubules were distorted and their lining
epithelium was destroyed. Interstitial edema was present.
However, there was no evidence of necrosis (Figure 3C).
The glomeruli were reduced in number. Sections from TDF treated rat kidney medulla showed mild
distortion
of renal tubular epithelium. There was a diffuse epithelial
cell shrinkage observed, suggestive of apoptotic changes
(Figure 3D). |
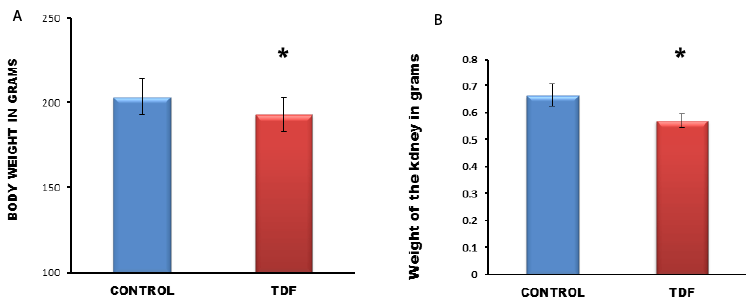 |
| Figure 2: Body weight and kidney weight of control rats and TDF treated
rats. Significant reduction in body weights and kidney
weights between control rats and TDF treated rats. Values represent mean ± S.D., n=6.
*P<0.05 vs. control. |
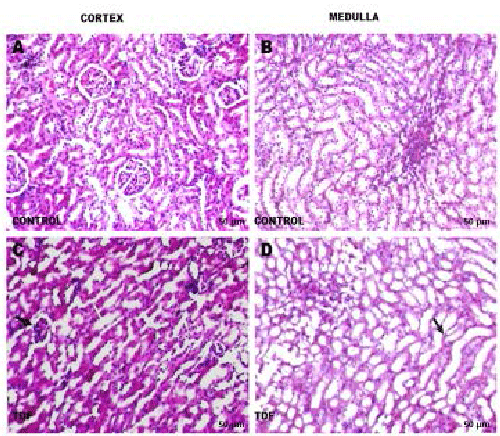 |
| Figure 3: Representative light micrographs of rat kidney. (A) Renal
cortex of a control rat-shows normal architecture [H& E,
200X]; (B) Renal medulla of a control rat shows normal architecture [H & E, 200X]; (C)
renal cortex of a TDF treated rat. The
proximal convoluted tubules were distorted and their lining epithelium was destroyed (white
arrow, H & E, 200X). Some glomeruli
were shrunken (black arrow); (D) Renal medulla of a TDF treated rat?There was mild
destruction of the lining epithelium of the
loops of Henle and the convoluted tubules (black arrow) H & E, 200X. |
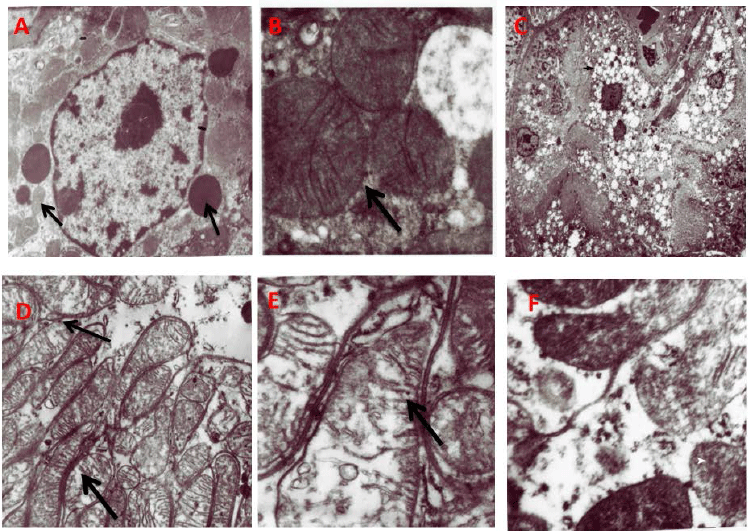 |
| Figure 4: Representative electron micrographs of tubular mitochondria. A)
Normal Kidney tubules (original magnification ×
22000); (B) Normal mitochondrial structure (black arrow) in the renal tubules of control
rats (original magnification × 22000);
(C) Vacuoles seen in the cytoplasm of the kidney tubule (black arrow) Less number of
lysosomes (white arrow); (D) Swollen
mitochondria; (M) black arrow(original magnification × 22000) E; Disruption of
mitochondrial cristae (black arrow) in the renal
tubules of TDF treated rats; (F) Amorphous deposits in the mitochondrial matrix (white arrow)
× 22,000. |
TDF treatment causes severe damage to the proximal
tubular mitochondria |
| Vehicle-treated rats showed normal tubular structure with
numerous mitochondria and lysosomes (black arrows)
(Figure 4A). Oval mitochondria with densely packed cristae
were observed (Figure 4B). Proximal tubular epithelia of
TDF-treated rats showed moderate to severe damage to the
mitochondria. The cytoplasm showed increased number
of vacuoles and reduced number of lysosomes. Nucleus
appeared shrunken (Figure 4C). The mitochondria showed
marked variations in size and shape. Mitochondrial
toxicity included swollen (giant) mitochondria (Figure
4D), disrupted cristae (Figure 4E), and accumulation of
amorphous deposits in the mitochondrial matrix (Figure
4F). An increase in the number of mitochondria with
irregular shape and fragmented cristae was observed in the
cytoplasm of basal part of tubule cell. In some epithelial
cells, the mitochondria were reduced in number. These
findings suggest that TDF targets mainly the proximal
tubular mitochondria and to a lesser extent the lysosomes
and nucleus. |
TDF treatment causes mitochondrial dysfunction |
| RCR was reduced by 44% in the kidneys of TDF-treated rats, suggesting mitochondrial
dysfunction (Figure
5). Decreased RCR indicates uncoupling of oxidative
phosphorylation and suggests extensive mitochondrial
damage. |
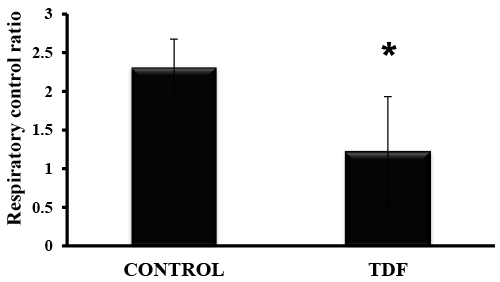 |
| Figure 5: RCR in the mitochondria isolated from TDFtreated
kidneys and control rat kidneys. Values represent
mean ± S.D., n=6. *P<0.05 vs. control. |
TDF treatment causes proximal tubular dysfunction -
acquired Fanconi syndrome |
| Chronic TDF treatment has been shown to induce
proximal renal tubular dysfunction that resembles Fanconi
syndrome (Table 1), which is characterized by increased
urinary losses of bicarbonate, phosphate, amino acids,
glucose and other nutrients due to decreased reabsorption
at the proximal renal tubuli. Proximal tubular function was
impaired in TDF-treated rats, as evidenced by increased
urinary excretion of phosphate, potassium and bicarbonate
and a considerable reduction in serum phosphate,
bicarbonate, and potassium. |
TDF causes tubular proteinuria |
| We found no proteinuria or glycosuria using dipstick. SDS
electrophoresis is a useful technique as it identifies low
molecular proteins i.e., tubular proteins with mol. wt. less
than 55,000 using a molecular weight marker protein. In
SDS-PAGE proteins are separated based on their molecular
weight. Individual proteins can then be identified within
these patterns. |
| Urine from normal rats when subjected to electrophoresis
yielded an identifiable protein band that corresponded to
approximately Mr.60, 000, suggestive of albumin (Figure
6). In addition, faint bands were also observed in some
controls corresponding to molecular weight less than 55
KDa. a1 microglobulin is also detectable in normal urine.
Thus, normal rats appear to excrete detectable amount of
albumin (by electrophoresis), and negligible amount of
low molecular weight tubular proteins in urine. |
| Tubular proteinuria is characterized by the dominant
excretion of low-molecular-weight proteins such as
alpha 1-microglobulin or retinol-binding protein (RBP),
which correlate better with the extent of tubulo-interstitial
damage than does the determination of total 24-h protein
levels. The urine protein pattern in the TDF treated rats
revealed at least two bands of molecular weight lesser than
55 KDa suggesting tubular dysfunction. |
 |
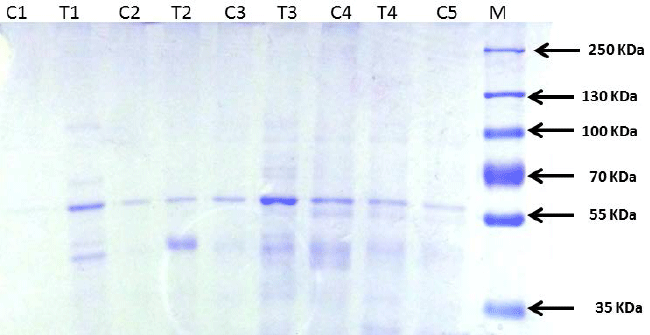 |
| Figure 6: Urine protein separation by SDS-PAGE
electrophoresis showing low molecular weight proteins (<55
KDa) in the TDF treated rats. |
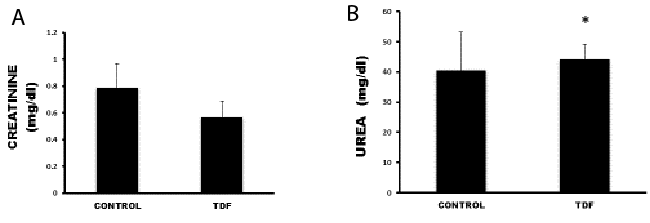 |
| Figure 7: Serum creatinine levels and urea levels in control rats and TDF
treated rats. Values represent mean ± S.D., n=6.
*P<0.05 vs. control. |
TDF causes no significant change in serum creatinine
levels but increases urea levels mildly |
| Serum urea levels were increased slightly in the TDF treated
rats as compared with control (Figure 7). TDF treatment
had no significant effect on the serum creatinine values. It
has been demonstrated that renal tubular dysfunction can
occur in patients with or without a decrease in glomerular
filtration rate [33] as decline of renal function is evident
only after persistent tubular injury. |
Discussion |
| Long term exposure to TDF is associated with an increased
risk over time of kidney tubular abnormalities in the
absence of significant impaired glomerular function (serum
creatinine, GFR etc.]. The main site of toxicity seems to
be the proximal tubule, and in more severe cases, patients
can develop Fanconi syndrome (which is characterized
by tubular proteinuria, aminoaciduria, phosphaturia,
glycosuria, and bicarbonate wasting [17] or acute kidney
injury. In order to investigate TDF nephrotoxicity a
reliable animal model is necessary. Although a few models
have been proposed earlier, to date there is no standard
animal model that is available for the study of mechanism
of TDF induced nephrotoxicity. The rat is proven to be a
useful model for the study of nephrotoxicity of a number
of agents, including gentamicin, an antibiotic, cisplatin, a
chemotherapeutic drug, and acetaminophen, an antipyretic
drug. Therefore, we chose to standardise a rat model of
TDF nephrotoxicity. |
| We tried different doses of TDF and different time period
of treatment. We tried 300 mg/60 kg body weight/day
(human dose) [3] for 5 weeks, 100 mg/kg body weight/
day for 8 weeks [21], 300 mg/kg body weight/day for 8
weeks [24], and finally 600 mg/kg body weight/day for
5 weeks. We could find proximal tubular damage and
dysfunction only in those rats that were treated with 600
mg/kg body wt. TDF/day. Histologically we were able
to observe proximal tubular damage, and mitochondrial
abnormalities as seen in human biopsies. Proximal
tubular function was also impaired in TDF-treated rats,
as evidenced by increased urinary excretion of phosphate, potassium and bicarbonate and a
considerable reduction in
serum phosphate, bicarbonate, and potassium. However,
there was no significant change in serum creatinine values
between TDF treated rats and control rats, suggesting
that glomerular functions were unaffected upon TDF
treatment, just as seen in humans on TDF therapy [33]. |
| In the present study we have used high dose of TDF (12
× human dose) to produce proximal tubular damage and
dysfunction in rats. The requirement of very high dose
of TDF to produce a rat model of TDF nephropathy may
be attributed to 1. Usage of normal rats instead of HIV
infected ones as HIV itself is known to affect the renal
functions in humans; 2. The administration of TDF only
and no other drug unlike humans who may be on other
antiretroviral drugs or any other drug that may affect
renal function or clearance; 3. Induction of renal tubular
toxicity within a short period of time. This may justify the
requirement of high dose of TDF in order to produce TDF
tubulopathy in rats. |
Conclusion |
| This rat model is a good model for the study of TDF
nephrotoxicity as it resembles that of TDF nephrotoxicity
in humans both structurally and biochemically. The model
will be useful not only for the study of nephrotoxicity
of TDF but also for carrying out intervention studies.
This model will also permit to study drug interactions
by overcoming the limitations of cell culture and the
difficulties of obtaining human kidney samples. A
limitation of this model in our opinion is the usage of 12 ×
dose of TDF used in humans in order to produce proximal
tubular nephropathy. |
Acknowledgments |
| We would like to thank the Centre for Scientific and
Industrial Research (CSIR), New Delhi for the financial
support. Ms. Hemalatha R. worked as a Senior Research
Fellow on the project. |
References |
- Gallant JE,
Deresinski S. Tenofovir disoproxil fumarate. Clinical Infectious Diseases 2003; 37: 944-950.
- Delaney WE 4th, Ray AS, Yang H, Qi X, Xiong S, Zhu Y, Miller MD. Intracellular metabolism
and in vitro activity of tenofovir against hepatitis B virus. Antimicrob Agents Chemother 2006;
50:2471-2477.
- Gilead Sciences Inc. Drug approval package for NDA 21?356: VIREAD (tenofovir disoproxil
fumarate). U S Food and Drug Administration FDA Report 2001.
- Squires K, Pozniak AL, Pierone G Jr, Steinhart CR, Berger D, Bellos NC, Becker SL, Wulfsohn
M, Miller MD, Toole JJ, Coakley DF, Cheng A. Tenofovir disoproxilfumarate in nucleoside-resistant
HIV-1 infection: a randomized trial.Ann Intern Med 2003; 139:313-320.
- Gallant JE, Staszewski S,Pozniak AL. Efficacy and safety of tenofovir DF vs stavudine in
combination therapy in antiretroviral-naive patients: a 3-year randomized trial JAMA 2004; 292:
191-201.
- Birkus G, Hitchcock M, Cihlar T. Assessment of mitochondrial toxicity in human cells
treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors.
Antimicrob Agents Chemother 2002; 46: 716-723.
- Malik A, Abraham P, Malik N. Acute renal failure and Fanconi syndrome in an AIDS patient
on tenofovir treatment-case report and review of literature. J Infect 2005; 51:E61-65.
- Peyriere H, Reynes J, Rouanet I.Renal tubular dysfunction associated with tenofovir
therapy: report of 7 cases. J Acquir Immune DeficSyndr2004; 35:269-273.
- Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-Associated Kidney Toxicity in
HIV-Infected Patients: A Review of the Evidence. Am J Kidney Dis 2011; 57:773-780.
- Breton G, Alexandre M, Duval X. Tubulopathy consecutive to tenofovir-containing
antiretroviral therapy in two patients infected with human immunodeficiency virus-1. Scand J
Infect Dis 2003; 36: 527-528.
- Perazella MA. Tenofovir-induced kidney disease: an acquired renal tubularmitochondriopathy.
Kidney Int 2010; 78: 1060-1063.
- Mocroft A, Kirk O, Gatell J. Chronic renal failure among HIV-1-infected patients. AIDS
2007; 21:1119?1127.
- Cote HC, Magil AB, Harris M. Exploring mitochondrial nephrotoxicity as a potential
mechanism of kidney dysfunction among HIV-infected patients on highly active antiretroviral
therapy. AntivirTher 2006; 11:79-86.
- Antoniou T, Raboud J, Chirhin S. Incidence of and risk factors for t enofovir-induced
nephrotoxicity: a retrospective cohort study. HIV Med2005; 6: 284-290.
- Rodriguez-Novoa S, Alvarez E, Labarga P, Soriano V. Renal toxicity associated with
tenofovir use. Expert Opin Drug Saf2010; 9:545-559.
- Karras A, Lafaurie M, Furco A. Tenofovir-related nephrotoxicity inhuman Immunodeficiency
virus-infected patients: three cases of renal failure, Fanconi’s syndrome and nephrogenic
diabetes insipidus. Clin Infect Dis 2003; 36:1070-1073.
- Quinn KJ. Incidence of proximal renal tubular dysfunction inpatients on tenofovir
disoproxilfumarate.Int J STD AIDS 2010; 21:150-151.
- Herlitz LC, Mohan S, Stokes MB, Radhakrishnan JD, Agati VD, Markowitz GS. Tenofovir
nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial
abnormalities. Kidney International 2010; 78: 1171?1177.
- Bianchi V. Nucleotide pool unbalance induced in cultured cells by treatments with
different chemicals. Toxicology 1982; 25:13-18.
- Mercy L, Pauw A, Payen L. Mitochondrial biogenesis in mtDNA-depleted cells involves a
Ca2+-dependent pathway and a reduced mitochondrial protein import. FEBS J 2005;
272:5031-5055.
- Lebrecht D, Venhoff AC, Kirschner J, Wiech T, Venhoff N, Walker UA.Mitochondrial
tubulopathy in tenofovir disoproxil fumarate-treated rats. Journal of Acquired Immune Deficiency
Syndromes 2009; 51: 258-263.
- Kohler JJ, Hosseini SH, Hoying-Brandt A, Green E, Johnson DM, Russ R, Tran D, Raper CM,
Santoianni R, Lewis W. Tenofovir renal toxicity targets mitochondria of renal proximal tubules.
Lab Invest 2009, 89:513-519.
- Dalakas MC, Illa I, Pezeshkpour GH. Mitochondrial myopathy caused by long-term zidovudine
therapy. N Engl J Med 1990; 322:1098-1105.
- Biesecker G, Karimi S, Desjardins J. Evaluation of mitochondrial DNA content and enzyme
levels in tenofovir DF-treated rats, rhesus monkeys band woodchucks. Antiviral Res 2003; 58:
217-225.
- JW Sons. Mitochondrial Dysfunction in Drug-Induced Toxicity.John Wiley & Sons,
Hoboken, NJ, USA 2008.
- Allen CT. Laboratory Methods: In: Histochemistry. 1st edE.B.Prophet co., American Registry
of Patholog 1992; pp.53.
- Lewis W, Grupp IL, Grupp G, Hoit B, Morris R, Samarel AM. Cardiac dysfunction occurs in
the HIV-1 transgenic mouse treated with zidovudine. Lab Invest 2000; 80:187-197.
- Trump BF, Berezesky IK,Laiho UK, Osornio AR, Mergner WJ, and. Smith MW.The role of calcium
in cell injury: a review. ScanningElectron Microsc1980; 2:437-462.
- Brocklebank T, Cooper EH, Richmond K. Sodium dodecyl sulphate polyacrylamide gel
electrophoresis patterns of proteinuria in various renal diseases of childhood.
PediatrNephrol1991; 5:371-375.
- Masola B, Evered DF. Preparation of rat enterocyte mitochondria Biochem J
1984;218:441-447.
- Nakatani T, Nakashima T, Kita T. Succinate dehydrogenaseactivities of fibers in the rat
extensor digitorumlongus, soleus, and cardiac muscles. Arch HistolCytol1999;62:393-399.
- Madesh M, Ramachandran A, Balasubramanian KA.Nitric oxide prevents anoxia-induced apoptosis
incolonic HT29 cells. Arch BiochemBiophys 1999;366:240-248
- Horberg M, Tang B, Towner W. Impact of tenofovir on renal function in HIV-infected,
antiretroviral-naive patients. J Acquir Immune DeficSyndr2010; 53:62-69.
|







