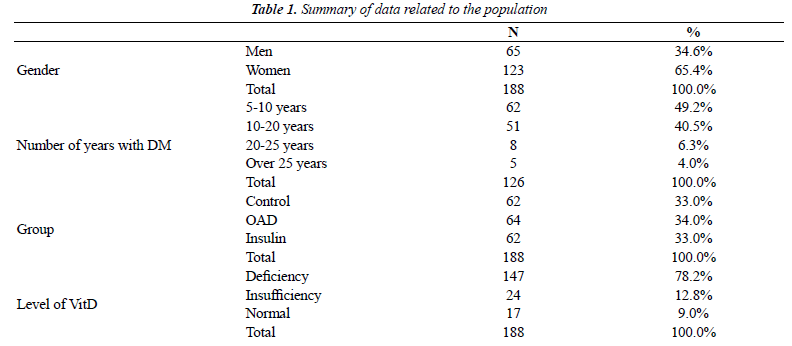Keywords
|
| Type 2 DM, Oral hypoglycemic agents (OHAs), Insulin, 25(OH) Vitamin D |
Introduction
|
| Diabetes mellitus (DM), a disease caused by absolute or relative insulin deficiency or insulin resistance, is manifested by hyperglycemia and characterized by carbohydrate, lipid, and protein metabolism disorder [1]. |
| The obese population has been increasing rapidly worldwide due to the fast development of unhealthy and irregular eating habits, as well as sedentary lifestyle, aging, and urbanization. On this matter, the International Diabetes Federation has estimated that the number of people with type 2 diabetes mellitus (T2DM) will increase to 334 million by 2025 [2]. |
| Chronic hyperglycemia in DM patients triggers damage to a variety of organs and associated dysfunctions; the affected organs include kidneys, nerves, heart, blood vessels, and mainly eyes. Acute metabolic complications as well as chronic microvascular and macrovascular complications are the most significant causes of morbidity and early mortality associated with DM [3,4]. |
| Vitamin D reduces insulin resistance and increases insulin sensitivity [5,6]. The aim of this study was to identify the relationship between the level of 25(OH) Vitamin D and T2DM. Distinctively from other similar studies, the level of 25(OH) Vitamin D in T2DM patients taking oral hypoglycemic agents (OHAs) will be compared with those taking insulin by means of metabolic parameters. |
Materials and Methods
|
| This study (approved by decision no. 12 of May 14, 2013 of the Ethics Board of the Faculty of Medicine, Eski?ehir Osmangazi University) is based on the data collected from 126 patients of T2DM who were admitted to the Polyclinic of Endocrinology in the Department of Internal Medicine and Policlinic of Family Medicine and 62 healthy controls admitted for periodic health examinations to the same Polyclinic in the Education and Research Hospital of Faculty of Medicine, Eski?ehir Osmangazi University between October 1, 2013 and April 30, 2014. In order to raise the data needed for this study, HbA1C, lipid profile, and 25(OH) vitamin D levels of the patients and the control subjects were determined simultaneously. The sociodemographic data related to the patients were recorded. |
| From 126 T2 DM patients, 64 were taking OHAs and 62 were on insulin treatment. Patients with type 1 and other subgroups of diabetes, treated in the diabetes policlinic, were excluded from the study. Only T2DM patients taking OHAs and insulin were included in the study. T2DM patients who presented chronic liver or kidney disease, or any parathyroid disease, i.e., conditions that are likely to affect the level of Vitamin D, were also excluded from the study. All patients presented the normal level range of ALT, AST, BUN, Cr, and parathormone. |
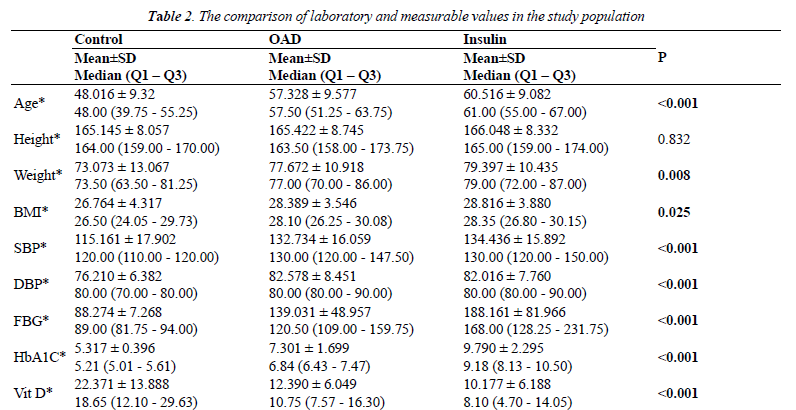
| The following factors were considered for the evaluation of patients: body mass index (BMI=kg/m2), diabetes onset date (based on the anamnesis obtained from patients), the fasting blood glucose levels (FBG), HbA1C, ALT, AST, BUN, Cr (creatinine), parathormone, Ca (calcium), P (phosphorus), and albumin were determined according to biochemical parameters. Ages of the patients used for control, OHAs, and insulin groups were 48.016 ± 9.32, 57.328 ± 9.577, 60.516 ± 9.082 years, respectively. |
| The control group subjects consisted of individuals with an average age of >35 years presenting normal biochemical values, who did not have any additional disease and did not take any additional drugs. |
| The levels of 25(OH) Vitamin D were divided into four categories [7]: |
| • 0–20 ng/ml 25(OH) Vitamin D deficiency |
| • 21–29 ng/ml 25(OH) Vitamin D insufficiency |
| • 30–150 ng/ml 25(OH) Vitamin D normal level |
| • over 150 ng/mg 25(OH) Vitamin D excess |
Statistical analysis
|
| The statistical analysis of the data was conducted in the Program in Biostatistics, Faculty of Medicine, Osmangazi University. The continuous data were presented in the form of mean ± standard deviation and the categorical data were presented in the form of percentage (%). The Shapiro–Wilk test was used to observe whether the data showed normal distribution. In the comparison of groups that showed normal distribution, independent samples t-test was performed for the cases in which there were two groups, and one-way ANOVA was used for the cases in which the number of groups was three and more. In the comparison of groups that did not show normal distribution, Kruskal–Wallis H test was used for the cases in which the number of groups was three. Spearman’s rank correlation coefficients were calculated to determine the level of correlation among variables. Pearson’s chi-squared and Pearson’s exact chi-squared tests were performed for the analyses of cross tables. IBM SPSS Statistics 21.0 software was used for data analyses. The value of p<0.05 was considered to be statistically significant. |
Results
|
| In total, the study was performed with 188 participants, from which 65 (34.6%) were males and 123 (65.4%) were females. Among the participants, 62 (33.0%) were in the healthy control group, 64 (34.0%) were in the group of DM patients taking OHA, and 62 (33.0%) were DM patients taking insulin. When considering the Vitamin D level variable, 147 (78.2%) individuals had Vitamin D deficiency, 24 (12.8%) had Vitamin D insufficiency, and 17 (9.0%) had normal level of Vitamin D. The characteristics of participants in this study are summarized in Table 1. |
| However, when considering the gender, there was no significant difference regarding the level of Vitamin D (χ2= 2.965 p=0.227). In the group of patients with Vitamin D deficiency, 48 (32.7%) were males and 99 (67.3%) were females; in the group of patients with Vitamin D insufficiency, 12 (50.0%) were males and 12 (50.0%) were females; and in the group of patients with normal Vitamin D level, 5 (29.4%) were males and 12 (70.6%) were females. |
| The average age of participants in the OHA group (57.328 ± 9.577) was higher than the average age of participants in the control group (48.016 ± 9.324). The average age of participants in the insulin group (60.516 ± 9.082) was higher than that of participants in the control group (48.016 ± 9.324). Considering the mean BMI values, the difference was also significant (KW=7.415; p=0.025). The mean BMI of participants in the OHA group (28.389 ± 3.546) was higher than the values for participants of the control group (26.764 ± 4.317). The mean BMI of participants in the insulin group (28.816 ± 3.880) was higher than that of participants in the control group (26.764 ± 4.317). When considering the systolic blood pressure (SBP) level, the values for participants in the OHA group (132.734 ± 16.059) was higher than those for the control group (115.161 ± 17.902). The SBP level was higher in the insulin group patients (134.436 ± 15.892) than the control group patients (115.161 ± 17.902). The diastolic blood pressure (DBP) level varied from 82.578 ± 8.451 in participants of the OHA group to 76.210 ± 6.382 in participants of the control group. The DBP level for participants in the insulin group (82.016 ± 7.760) was higher than that of participants in the control group (76.210 ± 6.382). The FBG factor values for participants in the insulin group (188.161 ± 81.966) were higher than that for participants in the OHA group (139.031 ± 48.957). The HbA1C level in the insulin group participants (9.790 ± 2.295) was higher than the HbA1C level in the OHAs group (7.301 ± 1.699). In the group of patients taking OHAs and insulin (i.e., the DM group), the difference in the HbA1C level was not significant (p>0.05) between the patients with BMI ≤ 30 (8.9503 ± 2.69014) and those with BMI >30 (8.3626 ± 2.21934). |
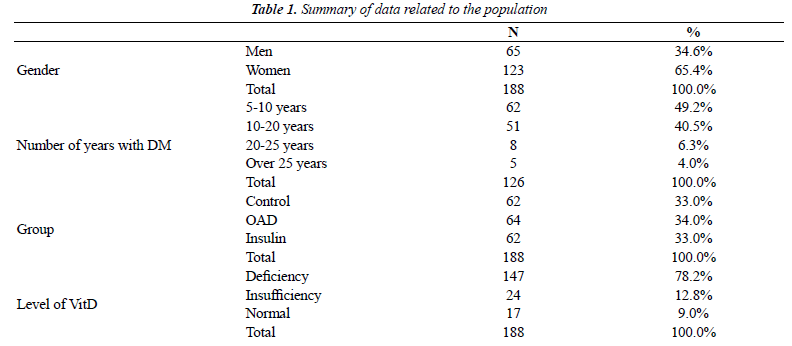 |
| The tests indicated that the participants’ levels of ALT, AST, BUN, Cr, PTH, Albumin, Ca, and P serum were within the normal range. The 25 (OH) vitamin D levels of participants in the control group (22.371 ± 13.888) were higher than those of participants in the OHA group (12.390 ± 6.049). The higher values of 25(OH) Vitamin D were shown for participants in the control group (22.371 ± 13.888) compared with the values for participants in the insulin group (10.177 ± 6.188). In the OHA group, 25(OH) Vitamin D level (12.390 ± 6.049) was higher than the values displayed by participants in the insulin group (10.177 ± 6.188); however, the difference was not statistically significant. The findings are summarized in Table 2. |
| As to the level of 25(OH) Vitamin D, there was no statistically significant difference in the whole group between participants with BMI >30 and those with BMI ≤ 30 (p>0.05). |
| The SBP level for participants with 25 (OH) Vitamin D deficiencies (129.524 ± 16.700) was higher than those for participants with 25(OH) Vitamin D at normal level (112.353 ± 30.929). The results of the Kruskal–Wallis H test, which was conducted to see whether the mean DBP of participants had significant difference regarding to the 25(OH) Vitamin D level, revealed no significant difference between the groups (p>0.05). |
| The FBG levels for participants with 25 (OH) Vitamin D deficiencies (148.612 ± 72.441) were higher than those for participants with 25 (OH) Vitamin D insufficiencies (111.458 ± 37.584). The FBG levels for participants with 25 (OH) Vitamin D deficiencies showed higher values (148.612 ± 72.441) than those for participants with normal 25(OH) Vitamin D level (89.177 ± 7.038). |
| In diabetic patients, the HbA1C level for participants with 25 (OH) Vitamin D deficiencies (7.891 ± 2.563) was higher than those for participants with 25 (OH) Vitamin D insufficiencies (6.385 ± 1.355). |
| When all participants were considered together, there was a significant reverse correlation at the level of 0.43 between HbA1C and 25(OH) Vitamin D (p<0.001). In patients taking OHA and insulin, there was a significant reverse correlation at the level of 0.25 between HbA1C and 25(OH) Vitamin D (p=0.004). |
 |
| The difference between the control, OHA, and insulin groups was not significant with regards to 25(OH) Vitamin D deficiency (the rate of VD deficiency was 82.3%, 71.9%, and 72.6%, respectively) (χ2= 2.273, p=0.321). |
Discussion
|
| DM is one of the most significant chronic health problems in the world. The world prevalence and incidence of DM vary considerably by regions. The difference in ethnicity and race plays an important role in this variation [4]. |
| It is known by a long time that Vitamin D deficiency constitutes a risk factor for impaired glucose tolerance. The level of Vitamin D is reported to be lower in T2DM patients than in non-diabetics. Researches also show that there is a positive correlation between Vitamin D level and insulin sensitivity in normal-weight individuals with normal glucose tolerance; low level of Vitamin D constitutes an independent risk factor for metabolic syndrome in a great part of population [5]. The level of Vitamin D was lower in patients with the risk of DM than in patients without risk of DM. Vitamin D deficiency is associated with impaired insulin secretion, which is a high risk factor for diabetes [6]. |
| In the present study, T2DM patients (those taking OHA drugs and those taking insulin) and a healthy control group were compared with regards to gender, age, BMI, year of diabetes start, HbA1C, FBG, and 25(OH) Vitamin D. Furthermore, all participants’ levels of ALT, AST, Ca, P, BUN, Cr, and albumin were determined for differential diagnosis of any other diseases (e.g., chronic liver disease, chronic kidney disease, and hyperparathyroidism) which are likely to affect the level of Vitamin D. These values were within the normal range in the patients included in this study. |
| In the group of patients, 62 (49.2%) had DM for 5–9 years, 51 (40.5%) for 10–19 years, 8 (6.3%) for 20–24 years, and 5 (4.0%) for 25 years or more. With respect to the 25(OH) Vitamin D level variable, 147 (78.2%) presented Vitamin D deficiency, 24 (12.8%) had Vitamin D insufficiency, and 17 (9.0%) had normal Vitamin D level. |
| In recent years, there has been greater focus on the relationship between Vitamin D deficiency and T2DM. Vitamin D was significantly lower in T2DM patients taking OHA and insulin compared with the control group. This result indicates that Vitamin D deficiency is associated with diabetes and glucose intolerance [8]. |
| Vitamin D plays an important role in beta cell functions, which is involved in the pathogenesis of insulin sensitivity, insulin resistance, and T2DM. Vitamin D (400–1000 U/ day) and calcium (600–1200 mg/day) support is important for the prevention of T2DM [9]. |
| In their clinical study, Newton et al. found a negative correlation between fasting blood glucose and level of Vitamin D in a group of African-American women [5]. Similarly, NHANES III analysis was conducted to identify the relationship between serum 25(OH) Vitamin D, race, and diabetes risk. It was found that insulin resistance was higher in non-Hispanic black people than in white people, and that the correlation between diabetes risk and Vitamin D level was reverse [10]. |
| There is an increased risk of T2DM in obese and overweight people. Obesity and overweight increase the relative risk of diabetes four times in individuals aged between 20 and 44 [11]. |
| In a cohort study, the diabetes risk showed very strong correlation with BMI in over fifty thousand American male employees; the risk also applies to women. The relative risk of T2DM was high in the 90th percentile of BMI among 43,581 women registered in Nurses’ Health Study [12]. High BMI is considered a dominant risk factor for T2DM. Haffner et al. observed 1,734 individuals for 7 years in the San Antonio Heart Study and reported that 195 individuals who developed T2DM had higher BMI values [13]. The results of many studies support that T2DM may be mostly prevented by the adoption of healthy lifestyle and treatment methods that reduce obesity. As it was the case in a number of epidemiological studies, the frequency of obesity was high among T2DM patients in our study. |
| In the present study, the difference in mean HbA1C levels was significant between the groups (KW=144.013; p<0.001). The results indicate that the HbA1C level was higher in the patients of the insulin group (9.790 ± 2.295) than in the OHA group (7.301 ± 1.699). |
| The results of our study showed that Vitamin D level was lower in patients of T2DM than in healthy control patients, as it was also suggested in many previous studies [14,15]. In patients of T2DM, the level of 25(OH) Vitamin D has reverse correlation with the HbA1C level. This correlation was observed in our study as well as in the large community-based study of Tromsö and the study of Hutchinson et al. [16]. |
| The main feature that distinguishes this study from others designed to disclose the relationship between 25(OH) Vitamin D level and T2DM on the basis of metabolic parameters is that we compared 25(OH) Vitamin D level between T2DM patients taking OHA and those taking insulin. We found that the level of 25(OH) Vitamin D was lower in the patients taking insulin, i.e., who had increased insulin resistance. Given that diabetes causes damage in several organs, as well as associated dysfunctions, it may be suggested that treatment with Vitamin D is a costeffective way to handle the disease. Even then, treatment using Vitamin D will be a long-term affair for T2DM patients. |
| |
References
- 1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008 ; 31: S55- 60.
- 2. Finch ZF, Zimmet PZ. Mortality from Diabetes. In: Alberti KGMMKrall LP (eds). The Diabetes Annual/4 Amsterdam: Elsevier, 1988.
- 3. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Commitee on the Diagnosis and Classification of Diabetes. Diabetes Care 2003; 26: 5-20.
- 4. Watkins PJ, Drury PL, Howell SL. Diabetes and its management 5th edn. Blackwell Co. 1996; pp. 3.
- 5. Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insülinresistance and β cell dysfunction. Am J Clin Nutr 2004; 79: 820-825.
- 6. Boucher BJ, Mannan N, Noonan K, Hales CN, Evans SJ. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia 1995; 38: 1239-1245.
- 7. Vitamin D Deficiency Medical Progress Michael F.Holick. The New England Journal Of Medicine 2007; 357: 266-281.
- 8. Alt?nova AE, Aktürk M, Törüner F, et al. The prevalence of vitamin d deficiency and its relationship with crp, fibrinogen, glycemic control and insulin resistance in patients with type 2 diabetesmellitus. Gazi Medical Journal 2010; 21: 117-120.
- 9. Özkan B, Döneray H. The non-skeletal effects of vitamin D. Çocuk Sa?l??? ve Hastal?klar? Dergisi 2011; 54: 99-119.
- 10. Joiner TA, Foster C, Shope T. The many faces of vitamin D deficiency rickets. Pediatr Rev 2000; 21: 296-302.
- 11. Vanitallie TB. Body weight, morbidity, and longevity. In: Bjorntorp P, Brodoff BN, eds. Obesity. Philadelphia, Pa: JB Lippincott Co; 1992.
- 12. Carry VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin dependent diabetes mellitus in women. The. Nurses Health Study Am J Epidemiol 1997; 145: 614-519.
- 13. Haffner SM , Mykkanen L, Festa A, et al. ?nsulin resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic state. Circulation 2000; 191: 975-980.
- 14. Isaia G, Giorgino R, Adami S. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care 2001; 24: 1496.
- 15. Need AG, O’Loughlin PD, Horowitz M, Nordin BEC. Relationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal women. Clinical Endocrinology 2005; 62: 738-741.
- 16. Hutchinson MS, Figenschau Y, Njølstad I, Schirmer H, Jorde R. Serum 25-hydroxyvitamin D levels are inversely associated with glycated haemoglobin (HbA1C). The Tromsö Study. Scandinavian Journal of Clinical and Laboratory Investigation 2011; 71: 399-406.
|