Case Report - Biomedical Research (2019) Volume 30, Issue 1
A case report of severe liver injury and hemophagocytic syndrome caused by chronic EB virus infection related to a mutation in the LYST gene.
Juan Kang1,2*, Weiqun Zeng1,2, Yixuan Yang1,2, Peng Hu1,2, Dazhi Zhang1,2, Zhi Zhou1,2, Dachuan Cai1,2, Yu Lei1,2 and Hong Ren1,2
1Chongqing City Center for the Study of Liver Disease Treatment, Institute for Viral Hepatitis, Key Laboratory of Molecular Biology for Infectious Diseases, Ministry of Education of China, China
2Department of Infectious Diseases, The Second Affiliated Hospital of Chongqing Medical University, 74 Linjiang Road, Yuzhong District, Chongqing, China
- *Corresponding Author:
- Juan Kang
Department of Infectious Diseases
The Second Affiliated Hospital of Chongqing Medical
University
China
Accepted date: January 25, 2019
DOI: 10.35841/biomedicalresearch.30-19-012
Visit for more related articles at Biomedical ResearchAbstract
Background: Hemophagocytic syndrome (HPS) is a syndrome of excessive immune activation characterized by various clinical symptoms of extreme inflammation. Epstein-Barr virus (EBV) is the most frequent infection associated with HPS, often referred to as virus-associated HPS. EBV-HPS is a serious disorder in which monoclonal growth of T cells infected with EBV and activated macrophages cause pancytopenia, high fever and severe liver injury due to primary infection or reactivation of EBV. Methods: A 22-year-old patient presenting with fever, pancytopenia and splenomegaly was diagnosed with hemophagocytic syndrome (HPS) associated with chronic Epstein-Barr virus (EBV) infection. The patient had been ill for more than one year and was more sensitive to glucocorticoid therapy. However, after tapering off the hormone therapy, the patient relapsed. Results: In view of the long-term glucocorticoid therapy of the patient, glucocorticoid-related side effects may have occurred. Therefore, intermittent VP-16 chemotherapy was given, and the glucocorticoid was reduced gradually to discontinuation. The patient, his brother and his parents had mutations in the HPS related genes. There was the same HPS gene mutation in the patient and his father (the variation c.4863-4 G>A (heterozygosity) in the introns around the LYST gene), and the same HPS gene mutation in the elder brother and mother (missense mutation c.603 G>T (p.Leu 201phe heterozygosity) in the Exon8 of STXBP2 gene). Therefore, there were no suitable sibling donors for the patient, who awaited an unrelated donor. It is presumed that the secondary HPS may have a genetic background. There have been HPS related gene mutations before symptom onset, and these can induce HPS due to infection and other factors. Conclusion: The detection of hemophagocytic related gene mutations for HPS high-risk populations with chronic EBV infection, tumors and autoimmune diseases can be beneficial in predicting the risk of HPS. STXBP2 gene mutations are common in patients with HPS, while LYST gene mutations are rarely reported. This case report provides some reference for the diagnosis of other patients.
Keywords
Hemophagocytic syndrome, Liver injury, EBV, LYST gene, STXBP2 gene
Introduction
Hemophagocytic syndrome (HPS) is a syndrome of excessive immune activation characterized by various clinical symptoms of extreme inflammation. The key pathophysiology of this syndrome is hyperactivation of cytotoxic T-lymphocytes and macrophages, as well as hypercytokinemia, resulting in hemophagocytosis, pancytopenia, high fever, severe liver injury, and splenomegaly [1-3]. HPS is classified into primary HPS and secondary HPS. The primary form of HPS shows clear familial inheritance or genetic causes, such as mutations in the PRF1, MUNC13-4 and STX11 genes [4-6]. Secondary HPS is associated with viral, bacterial, and fungal infections, as well as malignant tumors and rheumatologic disorders. Epstein-Barr virus (EBV) is the most frequent infection associated with HPS, often referred to as virus-associated HPS.
EBV-HPS is a serious disorder in which monoclonal growth of T cells infected with EBV and activated macrophages cause pancytopenia, high fever and severe liver injury due to primary infection or reactivation of EBV. Immunosuppressive therapies including administration of steroids are utilized as the primary treatment of EBV-HPS. As such, patients with severe HPS require allogeneic hematopoietic stem cell transplantation (allo-HSCT) therapy. We present here the case of a 22-year-old patient diagnosed with HPS associated with EBV infection. HPS in the Chinese Han population may be associated with an STXBP2 gene polymorphism [7]. However, in the presented case, there was a variation in the introns around Exon15 of the LYST gene in the patient, and the patient's brother and parents had an HPS related gene mutation that showed a familial gene mutation.
Case Presentation
The patient, a 22-year-old man, was admitted to the hospital on 2nd February 2018, suffering from eyelid edema for more than 1 year and fatigue for 3 weeks. For more than a year prior to admission, the patient had an eyelid edema without an obvious cause, starting from the left eyelid and developing into bilateral eyelids. There were no symptoms, such as eye pain, eyelid congestion, itching of the eyes, edema of the lower limbs, body yellow, poor appetite, fatigue, arthrodynia, photosensitivity, or rash in the patient. In the local hospital, the patient was treated with eye drops, to little or no effect.
Five months before admission to our facilities, the patient was hospitalized in the Pengshui County Hospital of Traditional Chinese Medicine. Abdominal ultrasonography showed that the liver was enlarged, the echo was changed, the portal vein system was widened, the gallbladder wall was thickened and crude, and with apparent splenomegaly. The hemogram exhibited that the concentration of hemoglobin was 153 g/L, the leucocyte count was 3.47 × 109/L, the percentage of neutrophils was 62.5%, and the total platelet count was 113 × 109/L. The liver function examination showed ALT 533 U/L, AST 371 U/L, GGT 159 U/L, ALP 470 U/L, ALB 39.7 g/L, Tbil 13.7 μmol/L, and Dbil 7.1 μmol/L. Coagulation function revealed PT 12.6 s and PTA 76.8%. The HBsAb and HBcAb were positive, and the antibodies of hepatitis A, hepatitis E and hepatitis C were negative. The patient was treated to protect the liver, and was released after slight improvement.
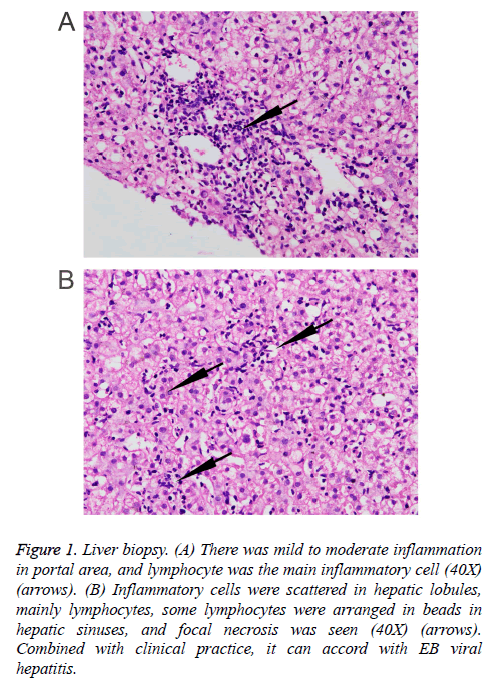
Three months prior to visiting our hospital, the patient felt more severe eyelid edema suffered from temperature fluctuation from 37°C to 38°C, but without chills, cough, expectoration, hemoptysis, chest pain, or oliguria symptoms. The patient was admitted to the Infection Department of the Southwest Hospital. At this time, abdominal ultrasonography revealed dense hepatic echo, thickened and edematous gallbladder wall, splenomegaly, widened splenic vein, and some fluid in the left chest cavity. A liver biopsy showed identifiable leaflet structure with 7-8 in the visual field, the hepatic cells were hydropic and denatured, there was fatty change in about 10% of the liver cells (large bubble type), and inflammatory cells, mainly lymphocytes, in the lobule, some lymphocytes were found arranged in a string of beads in the hepatic sinusoids, and focal necrosis was visible. Inflammation in the portal area was between mild and moderate, combined with the clinical information, the symptoms conformed to EB viral hepatitis (Figure 1). Tests for the IgG antibody of EB virus shell antigen and the IgG antibody of EBV were positive. EB virus PCR fluorescence showed 5.14 E+3 copies/ml. The hemogram showed that the concentration of hemoglobin was 142 g/L, the leucocyte count was 3.05 × 109/L, the percentage of neutrophils was 60.3%, and the total platelet count was 125 × 109/L. Liver function examination results showed ALT 475.5 U/L, AST 299 U/L, GGT 173 U/L, ALP 453 U/L, ALB 34.39 g/l, Tbil 11.38 μmol/L, Dbil 5.0 μmol/L, ceruloplasmin (CER) 40.7 mg/dl, transferrin (TRF) 206.0 mg/dl, and negative serum iron. Moreover, there were no obvious abnormalities in AFP, thyroid function, autoantibody, ANCA, antinuclear antibody, tuberculosis infected T cells, PPD skin test, renal function or electrolytes in this patient. The coagulation function showed PT 12.8 s and PTA 76.6%. The HBsAb and HBcAb were positive, while the antibodies of hepatitis A, hepatitis E and hepatitis C were negative. A marrow puncture observed the myeloid left shift, with visible eosinophils and the lymphoid cells showing abnormal morphology. The patient suffered from recurring fevers and was considered to have an EB virus infection. Therefore, the patient was treated with ganciclovir and liver protection. After treatment for a week, reexamination of liver function showed ALT 333.8 U/L, AST 211.7 U/L, GGT 167 U/L, ALP 450 U/L, ALB 34.48 g/L, Tbil 8.82 μmol/L, and Dbil 3.4 μmol/L. The patient released after a slight improvement, but he suffered recurrent double eyelid edema after discharge.
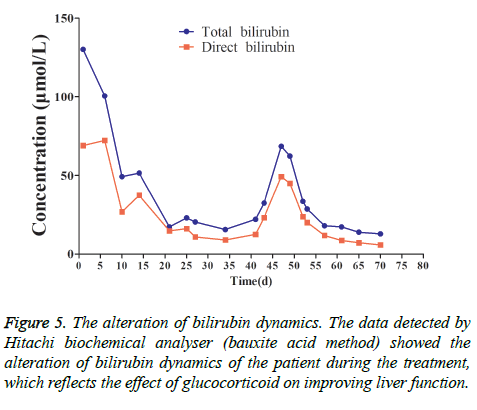
Figure 1: Liver biopsy. (A) There was mild to moderate inflammation in portal area, and lymphocyte was the main inflammatory cell (40X) (arrows). (B) Inflammatory cells were scattered in hepatic lobules, mainly lymphocytes, some lymphocytes were arranged in beads in hepatic sinuses, and focal necrosis was seen (40X) (arrows). Combined with clinical practice, it can accord with EB viral hepatitis.
Three weeks prior to admission in our hospital, the patient suffered from fatigue, poor appetite, nausea, abdominal distention, gingival bleeding, epistaxis and twitching of the hands and feet, jaundice without obvious causes.
Three days before admission, the patient developed vomiting and diarrhea, accompanied by fever, cough with a small amount of yellow and sticky sputum. He was hospitalized in the Respiratory Medicine Department of Qianjiang Central Hospital. In this hospital, a chest CT was performed that showed lung infection and bilateral pleural effusion. Liver function tests resulted in ALT 340 U/L, AST 544 U/L, GGT 384 U/L, ALP 1006.2 U/L, ALB 27.1 g/L, Tbil 105.0 μmol/L, Dbil 88.4 μmol/L, TBA 185.34 μmol/L and PCT 0.31 ng/ml. However, the patient did not improve, so was admitted to our hospital for further treatment. The patient lost 6 kg of weight since the onset of the disease.
In our hospital, the physical examination of the patient showed the body temperature was 38.3°C, the heart rate was 109 bpm, breathing was 20 breaths/min, and blood pressure of 106/76 mmHg. Moreover, the patient had a clear mind, but also had thin body, bilateral swelling of the eyelids, congestive and bleeding conjunctiva, symmetric and weakened bilateral tactile fremitus, percussive double lower lungs with wet rales, a flat abdomen without tenderness, a palpable spleen at 4 cm under the ribs, negative shifting dullness, and lower limbs with pitting edema of the second degree. In addition, an enhanced upper abdominal MR scan revealed hepatic interstitial edema, abnormality of hepatic parenchymal artery phase that was considered a response to inflammation, splenomegaly, a small amount of effusion in the abdominal cavity, and bilateral pleural effusion with a medium degree of effusion accompanied by segmental compression on the lower lobe of the lung. The chest CT found a large amount of effusion and some part parceled in the right thoracic cavity, and compulsive atelectasis in the partial right middle and lower lobes. Small and moderate amounts of effusion in the left thoracic cavity and multiple inflammation sites in the lung lobes, which were particularly obvious in the middle and lower lobes of the right lung, were also revealed by CT. Examination results are shown in Table 1.
| Liver function | ALT/AST | TB/DB | ALB |
|---|---|---|---|
| 316/460 U/L↑ | 130.2/89.1 μmol/l↑ | 25 g/L ↑ | |
| Hemogram | WBC | PLT | RBC |
| 2.29 × 109/L | 52 × 109/L | 5.54 × 1012/L | |
| HBV | HbsAg/anti-HBs | HbeAg/anti-Hbe | anti-Hbc |
| 0.637 COI/313.600 IU/L | 0.113 COI/1.410 COI | 0.008 COI | |
| EB-DNA | Blood | Lymphocyte | Pharynx |
| 3.37 × 104 copies/ml↑ | 8.27 × 104 copies/ml↑ | 6.75 × 103 copies/ml↑ | |
| Blood fat | Triglyceride | Cholesterol | |
| 3.1 mmol/L↑ | 5.1 mmol/L | ||
| Hepatitis | Hepatitis A IgM | Hepatitis E IgM | Hepatitis C IgM |
| Hepatitis C IgM | Negative | Negative | Negative |
| Protein | IL-2R | IL-2R | IL-2R |
| 2513 U/ml ↑ | 23.3 pg/ml↑ | 0.212 mg/ml | |
| Immunoglobulin | Ceruloprotein | ||
| Negative | Negative | ||
| Antibody | Anti-distrand-DNA antibody | Anti-distrand-DNA antibody | |
| Negative | Negative | ||
| Antibody spectrum of vasculitis | HDV antibody | ||
| Negative | Negative | ||
| Other | Blood ammonia | PTA | |
| 37 umol/L | 50% | ||
| Thyroid function | 9 pathogens in respiratory tract | ||
| Negative | Negative |
Table 1: The results of auxiliary examination.
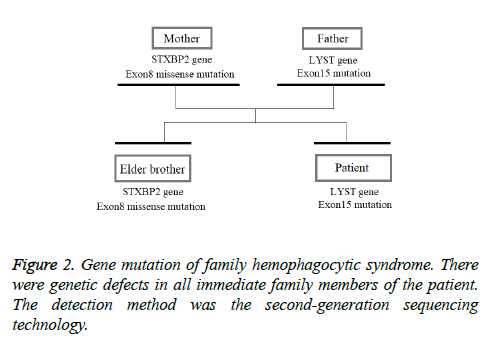
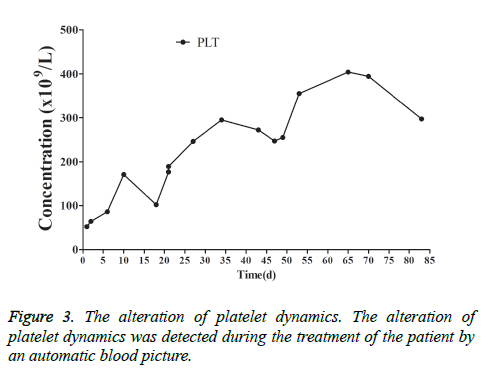
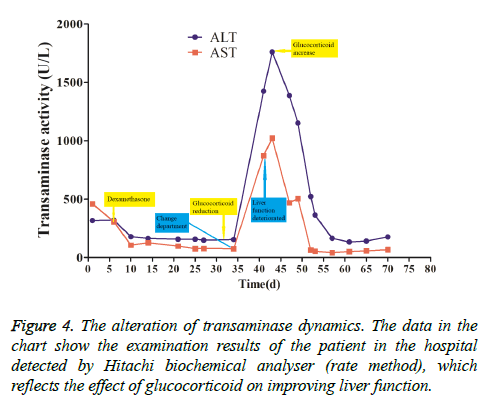
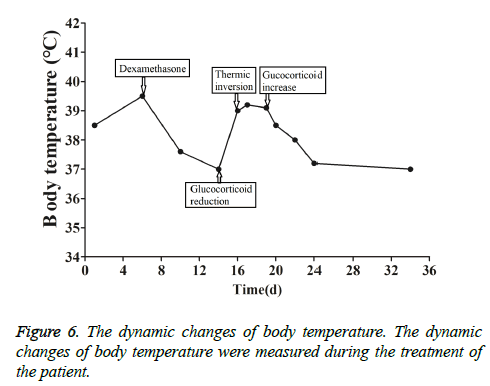
This patient was treated with piperacillin sulbactam to combat infection, received anti-viral treatment with acyclovir, and was treated for liver protection, inflammation and jaundice. However, five days after treatment, the patient still had recurrent fever, with the highest body temperature measured at 39.5°C, which was not significantly better than prior to admission. Moreover, although hemophagocytic cells were not found in the bone marrow examination, there was considerable concern about HPS in light of the patients' pancytopenia, fever, hepatomegaly, high blood lipid, elevated ferritin, and higher cytokine levels. Therefore, after consultation with the Department of Hematology, on the fifth day after admission the patient was treated with intravenous dexamethasone 15 mg once daily and immunoglobulin by implosive therapy for four days. Afterwards, the patient received I.V. dexamethasone 10 mg once daily by intravenous injection for four days and oral methylprednisolone (24 mg) for seven days. However, the patient developed fever again on February 18, 2018. Therefore, the fever was considered to be correlated with glucocorticoid reduction, and the dexamethasone treatment was continued for an additional 15 days at 10 mg per dose from February 20th onward. Fortunately, the patient showed significantly improved liver function, displayed no fever and the platelet count returned to normal. On March 7, 2018, the patient was transferred to the Department of Hematology and was recommended to receive allogeneic stem cell transplantation, bone marrow matching and HPS-related gene mutation detection (including the patient, his older brothers and parents). However, during treatment, the dexamethasone dosage was adjusted to 5 mg once daily for 10 days, and liver function deteriorated considerably. When the dexamethasone was adjusted to 10 mg once daily for 4 days, liver function did not obviously improve. The dexamethasone dosage was adjusted again to 15 mg once daily for 14 days, and the liver function gradually improved. The HPS genes of the patient's brother and mother showed that the missense mutation c.603 G>T (p. Leu201phe heterozygosity) existed for the STXBP2 gene Exon8. The patient's HPS genes showed the introns surrounding the LYST gene Exon15 had the variation c.4863-4 G>A (heterozygosity), and the HPS genes of the patient's father showed there is a variation of c.4863-4 G>A (heterozygosity) on the introns surrounding the LYST gene Exon15 (Figure 2). As shown in Figure 2, there were genetic defects in both the elder brother and the parents making them susceptible to HPS development and, thus, they were not suitable as stem cell donors. The patient was discharged on April 13, 2018, after mild and stable improvement, with regular follow-up checks. On April 26, 2018, re-examination showed WBC 3.07 × 109/L and PLT 297 × 109/L (Figure 3) and liver function showed ALT/AST 76/34 U/L (Figure 4), TB/DB 12/5.6 (Figure 5), and ferritin 23.8 ng/ml. The dynamic changes of liver function, platelets and body temperature during hospitalization are shown in Figure 6. In view of the use of large doses of glucocorticoid over 4 months, the patient might suffer from many complications such as infection, Cushing syndrome and other hormone related complications. Therefore, the patient began VP-16 chemotherapy prior to January. The glucocorticoid was gradually decreased and discontinued on June 20, 2018. However, the Bone Marrow Bank Centre continued to look for donors, and the patient is waiting to perform allogeneic hematopoietic stem cell transplantation.
Discussion
The young man was admitted to hospital due to suffering from eyelid edema for a year and fatigue for three weeks. The disease had a slow onset and long course with recurrent eyelid edema, severe injury of liver function, fever, hepatomegaly, conjunctival congestion, positive EB antibody and EB-DNA. The hepatoviruses, including hepatitis B, hepatitis C, hepatitis A and hepatitis E, and alcohol, drugs and poisons were excluded for the causes of his liver injury, and autoimmune liver diseases and inherited metabolic diseases were excluded. A liver biopsy in another hospital showed that there were spotty and fragmentary necrosis in the liver tissue, and there were many inflammatory cells (mainly mononuclear cells and lymphocytes) infiltrating around the necrotic foci and portal areas (three), and the fibrous hyperplasia was not obvious, which conformed to moderate chronic hepatitis. Therefore, it was diagnosed as non-hepatophilic viral hepatitis caused by chronic EB virus infection.
HPS, also known as hemophagocytic lymphohistiocytosis (HLH), is a reactive hyperplasia of the mononuclear- phagocytic system caused by a variety of pathogenic factors, accompanied by the active phenomenon of devouring one’s own blood cells, causing severe inflammatory reactions [8]. Persistent fever is often the most common symptom of this disease with the body temperature around 39°C. Other symptoms include hepatomegaly, splenomegaly, respiratory symptom, superficial lymphadenectasis, jaundice, rash, serous cavity effusion, skin ecchymosis or blood spots, central nervous system symptom, and renal function damage.
HPS is characterized by its complex clinical manifestations, rapid progression, high mortality and rarity [9]. The diagnostic criteria of HPS were referred from the diagnostic criteria of the HLH-2004 program developed by the Histolocyte Society in 2004. If the symptoms conform to five of the following eight indicators, HPS can be diagnosed: 1. Fever, lasting more than 1 week, peak>38.5°C. 2. Splenomegaly (above 3 cm in the subcostal region). 3. Hemocyte depletion (affects 2 or 3 series peripheral blood cells fevering: lasting more than 1 week, temperature peak>38.5°C): Hemoglobin<90 g/L (newborn: hemoglobin<100 g/L), platelets<100 × 109/L, neutrophil<1.0 × 109/L. 4. Hypertriglyceridemia and/or hypofibrinogenemia: fasting triglycerides ≥ 3.0 mmol/L, fibrinogen ≤ 1.5 g/L. 5. Histiocytosis and hemophagocytic phenomena without evidence of malignant diseases found in bone marrow, spleen, cerebrospinal fluid or lymph nodes. 6. NK cell activity is reduced or lacking (according to local laboratory indicators). 7. Serum ferritin ≥ 500 μg/L. 8. Plasma sIL-2R ≥ 2400 U/ml. The patient was infected by chronic EB virus, and suffered from continuous fever, a decrease in three blood cell lines, splenomegaly, hypertriglyceridemia, high ferritin, and sIL-2R significantly increased following the progress of the disease. Therefore, diagnosis as chronic EB-infection related HPS was warranted.
EB virus is a human herpes virus with a global distribution. EB virus infection can involve all systems of the body. The clinical manifestations are complex and diverse, and infectious mononucleosis is a common clinical presentation. The course of a few infectious mononucleosis cases can be deferred for months, and even years, known as chronic active EB virus infection. The EB virus is closely related to the occurrence of nasopharyngeal carcinoma and children's lymphoma. It is listed as one of the possible carcinogenic human tumor viruses. Severe EB virus infection cases may result in HPS, causing the death of the patient. Therefore, vigilant attention to the serious consequences caused by the chronic and protracted course of diseases after EB virus infection is prudent. HPS is divided into primary and secondary types. Primary HPS is from autosomal or sex-chromosome recessive inheritance, mainly occurring in children, with a poor prognosis [10]. Secondary HPS occurs mostly in adults, and most frequently seen in viral infection, especially in EB virus infection. Secondary HPS also occurs in malignant tumors, rheumatic immune disease, and organ transplantation. The mortality rate is from 20% to 70% [11]. Abnormal immune regulation plays a central role in the pathogenesis of HPS. Infection and other factors act on the organism, resulting in excessive activation and dysregulation of T lymphocytes and mononuclear phagocytes, and secretion of many inflammatory cytokines. The clinical manifestations of HPS are caused by inflammatory cell infiltration and hypercytokinemia. The activated inflammatory cells infiltrate the liver, spleen, lymph nodes, bone marrow and central nervous system and cause tissue damage. Cytokines induce fever, and the hemophagocytic phenomenon causes hematocytopenia. Tumor necrosis factor causes a decrease in lipoprotein activity leading to hypertriglyceridemia, while activated macrophages express fibrinolytic enzyme primary activation factor, resulting in hypofibrinogenemia. In laboratory examinations, impaired liver function is a prominent manifestation of this disease, often characterized by sharp deterioration of liver function and even liver failure, which may be associated with massive infiltration of inflammatory cells in the liver and secretion of large amounts of interferon gamma. HPS progresses rapidly and has a high mortality rate. It leads to higher mortality when the disease progresses to the stage of hemophagocytosis found in the bone marrow. At present, the treatment guidance for HPS is based mainly on the treatment standard recommended by HLH-2004: immunotherapy combined with chemotherapy. For secondary HPS treatment, it is necessary to first clear the cause, and then provide symptomatic treatment. The remission of primary disease has positive significance for the treatment of HPS. Hormone therapy alone has some effect on secondary HPS, but it should be adjusted into VP-16-based chemotherapy in a timely manner for patients insensitive to hormone therapy. Our patient had been infected with the EB virus for an extended period, and the disease was well progressed, which led to severe liver injury and HPS. When hemophagocytosis was not found in the bone marrow of the patient, we treated with glucocorticoids combined with human immunoglobulin and the patient improved, with the liver function gradually improving and the body temperature gradually returning to normal. However, when the glucocorticoid treatment was reduced, the patient recurred fever and liver injury, which suggests that the reduction of glucocorticoid should not be too fast, and clinicians must observe the patient's liver function, blood routine, body temperature closely during glucocorticoid reduction. Meanwhile, we investigated hemophagocytic related gene mutations in the patient and three other immediate family members, and all showed the mutations. Therefore, it is speculated that there is a genetic background in secondary HPS and a mutation of hemophagocytic related genes before onset, and HPS can be induced by infection and other factors.
For high-risk HPS groups with chronic EB infection, cancer and autoimmune diseases, evaluation of hemophagocytic related gene mutations is helpful to predict the risk of HPS [12].
Conclusion
HPS has poor prognosis, so early identification, positive and accurate treatment, timely consultation with the Department of Hematology and multi-departmental collaboration are of great significance for improving the prognoses of patients.
Data Availability
All data generated or analysed during this study are included in this published article.
Funding Statement
This study was supported by the National Natural Science Foundation of China Youth Fund (No. 81501482), National High-Quality Resource Sharing Course Fund ((2013) 115) and National High-Quality Course Fund ((2009) 217), Chinese Foundation for Hepatitis Prevention and Control (No. TQGB20190110).
Acknowledgement
We thank Professor Lin Chen and Dr Hanqing Zeng from Department of Hematology to provide the treatment information of the patient.
Informed Consent
The informed consent was signed by the patient. The study was approved by the Ethics Committee of The Second Affiliated Hospital of Chongqing Medical University (CMU: No.2018-1008-05).
References
- Filipovich A, McClain K, Grom A. Histiocytic disorders: recent insights into pathophysiology and practical guidelines. Biol Blood Marrow Transplant 2010; 16: S82-S9.
- Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med 2012; 63: 233-246.
- Takuro K, Noriaki K, Kiyoshi Y, Kikuchi I. Cord blood transplantation following reduced-intensity conditioning for Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis during systemic lupus erythematosus treatment. J Clin Exp Hematop 2016; 56: 126-129.
- Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA. Perforin gene defects in familial hemophago-cytic lymphohistiocytosis. Science 1999; 286: 1957-1959.
- Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell 2003; 115: 461-473.
- zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, Henter JI. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet 2005; 14: 827-834.
- Yang L, Tang Y, Xiong J, Liu YN, Zhang W, Xiao M. Hemophagocytic lymphohistocytosis in the Chinese Han population may be associated with an STXBP2 gene polymorphism. PLoS One 2016; 11: 0159454.
- Sadaat M, Jang S. Hemophagocytic lymphohistiocytosis with immunotherapy: brief review and case report. J Immunother Cancer 2018; 6: 49.
- Gupta S, Weitzman S. Primary and secondary hemophagocytic lymphohistiocytosis: clinical features, pathogenesis and therapy. Expert Rev Clin Immunol 2010; 6: 137-154.
- Shimada A, Kato M, Tamura K, Hirato J, Kanegane H, Takechi Y. Hemophagocytic lymphohistiocytosis associated with uncontrolled inflammatory cytokinemia and chemokinemia was caused by systemic anaplastic large cell lymphoma: a case report and review of the literature. J Pediatr Hematol Oncol 2008; 30: 785-787.
- Imashuku S. Clinical features and treatment strategies of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Crit Rev Oncol Hematol 2002; 44: 259-272.
- Chen X, Wang F, Zhang Y, Teng W, Wang M, Nie D. Genetic variant spectrum in 265 Chinese patients with hemophagocytic lymphohistiocytosis: Molecular analyses of PRF1, UNC13D, STX11, STXBP2, SH2D1A, and XIAP. Clin Genet 2018.