Case Report - Journal of Pathology and Disease Biology (2017) Journal of Pathology and Disease Biology (Special Issue 1-2017)
A case of heavy chain deposition disease complicated by acquired angioedema.
Rafia Chaudhry1*, Gautam Bhave2, Rachel Fissell2, Neil Sanghani2 and Paisit Paueksakon3
1Division of Nephrology and Hypertension, Albany Medical Center, Albany, NY, USA
2Division of Nephrology and Hypertension, Vanderbilt University Medical Center, Nashville, TN, USA
3Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
- *Corresponding Author:
- Rafia Chaudhry, MD
Division of Nephrology and Hypertension
Albany Medical College, USA
E-mail: chaudhr@mail.amc.edu
Accepted on January 25, 2017
Citation: Chaudhry R, Bhave G, Fissell R, et al. A case of heavy chain deposition disease complicated by acquired angioedema. J Pathol Dis Biol. 2017;1(1):1-5
Abstract
Heavy Chain Deposition Disease is a rare Monoclonal Immunoglobulin Deposition Disease presenting with proteinuria, hypertension and renal failure. Pathogenesis involves clonal expansion of B cells secreting free Ig heavy chains that deposit in the kidney. Diagnosis requires renal biopsy, where immunofluorescence detection of monoclonal heavy chains in the absence of light chains is pathognomonic. Treatment aims to suppress B cell production of the free heavy chains or eliminate abnormal B cell clone. We report an unusual case of HCDD with acquired angioedema, and postulate that free heavy chains may block C1 esterase inhibitor function, predisposing to bradykinin mediated angioedema.
Keywords
Acquired angioedema, Heavy chain deposition disease (HCDD), Immunoglobulin G3 (igg3).
Introduction
Heavy Chain Deposition Disease (HCDD) is rare, and presents with renal failure, proteinuria, hematuria, hypertension, and often hypocomplimenteniemia [1-3]. HCDD may mimic the pathology of immune complex, crescenteric glomerulonephritis, with isolated heavy chain deposits along mesangial, glomerular, and tubular Basement Membranes (BMs), without associated light chains [4]. The predominant heavy chain subtype is gamma. Rare cases of alpha and mu subtypes have also been reported [4,5]. Immunofluorescence for heavy chains alone is diagnostic. Renal pathology includes mesangial matrix expansion, hypercellularity, and nodular sclerosis resembling diabetic glomerulosclerosis. Fewer than 40 cases have been reported. We report a case of HCDD with the novel complication of acquired angioedema.
Case Report
Clinical history
A 45 yo male presented to the Emergency Room with dyspnea, orthopnea, fatigue, and lower extremity edema.
Medical history included hypertension, treated with lisinopril. Two weeks after initiating lisinopril, the patient developed angioedema, and lisinopril was stopped. Serum Cr (Scr) was 1.50 mg/dL.
The patient presented with hypertension (BP 200/129 mm Hg), coarse crackles at bilateral lung bases, jugular venous distention (10 cm), and 2+ bilateral lower extremity edema. Laboratory studies are given in Table 1. He developed a second episode of angioedema during his current course of illness, and this was 2 months after the patient had been off lisinopril.
| Laboratory Values | Day 1 | Day 3 | 3 months | 18 months |
|---|---|---|---|---|
| Serum Studies | ||||
| Sodium (meq/L) | 136 | 134 | 140 | 139 |
| Potassium (meq/L) | 4.6 | 4.6 | 4.0 | 4.0 |
| Serum urea nitrogen (mg/dL) | 37 | 41 | 34 | 13 |
| Creatinine (mg/dL) | 1.9 | 2.2 | 1.5 | 1.0 |
| Estimated GFR (mL/min/1.73 m2) | 46 | 39 | 61 | > 60 |
| Glucose (mg/dL) | 105 | 97 | 94 | 96 |
| Protein, total (g/dL) | 5.0 | - | 4.7 | 6.5 |
| Serum Albumin | 2.5 | - | 2.6 | 3.8 |
| Complement C3 (mg/mL) | 60 | - | 59 | 88 |
| Complement C4 (mg/mL) | 11.7 | - | 11.4 | 22 |
| CH50 (mg/dL) | ||||
| Cryoglobulin | ||||
| ANCA C-ANCA P-ANCA |
< 1:20 < 1:20 |
|||
| Anti-GBM antibody | ||||
| Antinuclear antibody | ||||
| Anti-dsDNA antibody (IU/mL) | 2 | |||
| Anti-Smith antibody | ||||
| Pro BNP(ng/dL) | 5800 | |||
| Hepatitis B surface antigen | nonreactive | |||
| Hepatitis C antibody | reactive | |||
| Hepatitis C PCR | not detected | |||
| HIV antibody | nonreactive | |||
| Rheumatoid Factor | <8.6 | |||
| Hematologic Studies | ||||
| Hemoglobin (g/dL) | 10.5 | 10.1 | 8.7 | 12.6 |
| White blood cell count (x103/μL) | 8.2 | 7.44 | 6.7 | 3.8 |
| Platelet count (x103/μL) | 269 | 334 | 193 | 226 |
| Urine Studies | ||||
| pH | 7.5 | 5.5 | ||
| Specific gravity | 1.020 | 1.021 | ||
| Urine protein (mg/dL) | >1000 | 100 | ||
| Red blood cells (/HPF) | 63 | 5 | ||
| White blood cells (/HPF) | 9 | 1 | ||
| Red blood cell casts | none | |||
| Dysmorphic red blood cells | none | |||
| Protein: creatinine ratio (mg/mg) | 2.0 | |||
| 24-h urine protein | 3619.5 | 1951 | ||
Table 1. Laboratory studies at initial presentation and subsequent follow up: GFR: Glomerular filtration rate; ANCA: Antineutrophil cytoplasmic antibody; dsDNA: Double stranded DNA; GBM: Glomerular basement membrane; BNP: Brain natriuretic peptide; HPF, high power field.
Trans-thoracic echocardiogram demonstrated left ventricular hypertrophy, with heavy trabeculations, ejection fraction of 41%, and moderately enlarged left atrium. Cardiac MRI failed to meet criteria for non-compaction cardiomyopathy and amyloidosis.
Hepatitis C antibody was positive, complement levels were decreased (C3 60 mg/dL, C4 11.7 mg/dL), and other serologies negative (Table 1).
Patient symptoms improved with diuresis, renal function declined, and a left percutaneous renal biopsy was performed.
Kidney biopsy
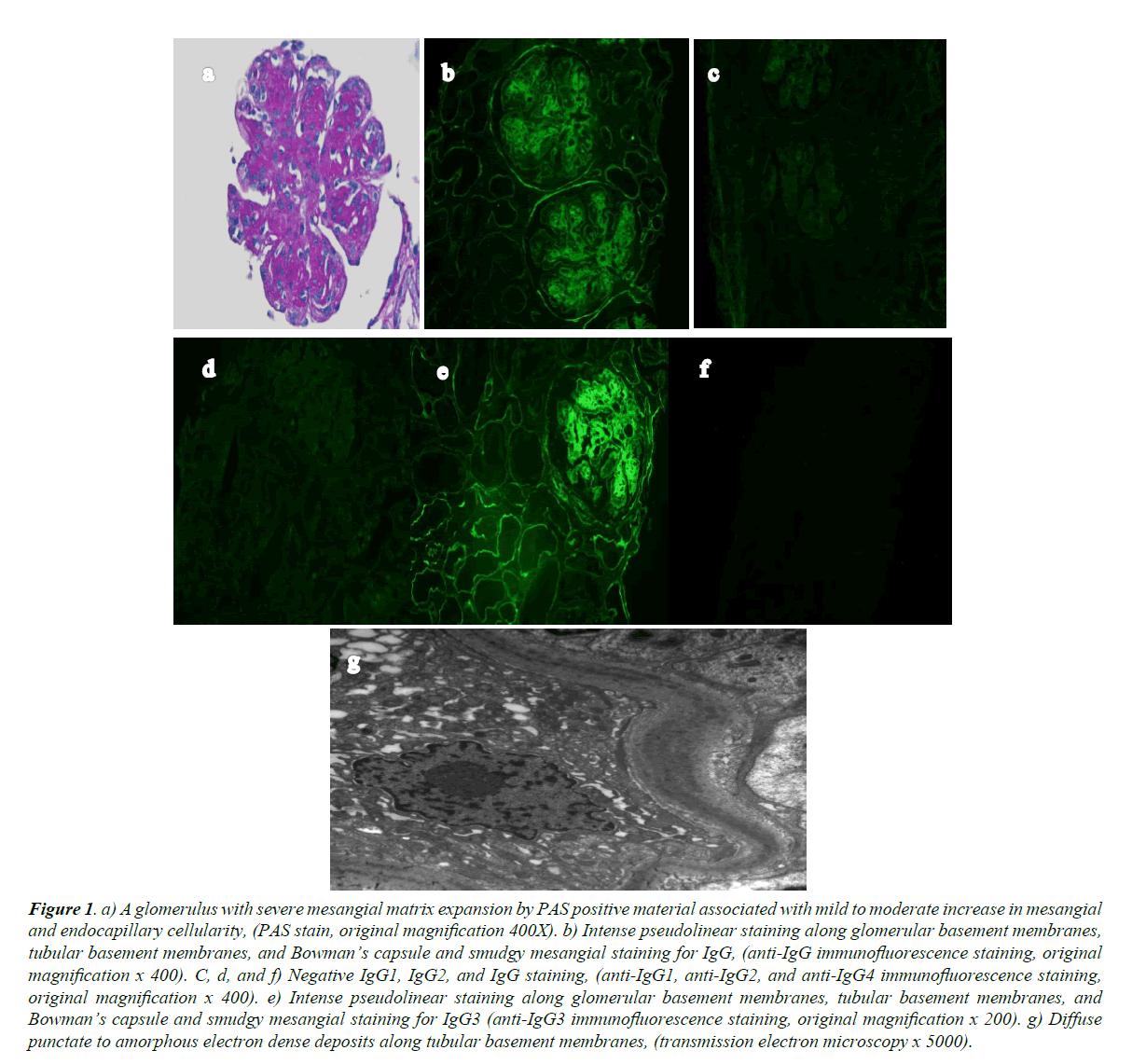
Light microscopy showed eighteen glomeruli. One was globally sclerosed, seventeen had diffuse, global, nodular matrix expansion, marked mesangial hypercellularity, obliteration of the capillary lumina by endocapillary hypercellularity, and extensive eosinophilic, strongly PAS-positive material (Figure 1a). There was diffuse splitting of the glomerular BMs, and two glomeruli with crescents; one fibrocelllular, and one cellular with associated adhesion to Bowman’s capsule and GBM break. There was 10% interstitial fibrosis, without interstitial inflammation. Arteries were unremarkable.
Five glomeruli in frozen sections were processed for immunofluorescence. There was 3+ (0 to 3+ scale) pseudolinear capillary loop, Bowman’s space, and tubular BM staining with smudgy mesangial IgG staining (Figure 1b). There was 1+ smudgy mesangial staining for C3 and C1q. IgA, IgM, kappa, and lambda were negative. IgG subclass staining showed 3+ pseudolinear capillary loop, Bowman’s space, and tubular BM staining with smudgy mesangial staining for IgG3 (Figure 1e). IgG1 (Figure 1c), IgG2 (Figure 1d), and IgG4 (Figure 1f) were negative.
Figure 1. a) A glomerulus with severe mesangial matrix expansion by PAS positive material associated with mild to moderate increase in mesangial and endocapillary cellularity, (PAS stain, original magnification 400X). b) Intense pseudolinear staining along glomerular basement membranes, tubular basement membranes, and Bowman’s capsule and smudgy mesangial staining for IgG, (anti-IgG immunofluorescence staining, original magnification x 400). C, d, and f) Negative IgG1, IgG2, and IgG staining, (anti-IgG1, anti-IgG2, and anti-IgG4 immunofluorescence staining, original magnification x 400). e) Intense pseudolinear staining along glomerular basement membranes, tubular basement membranes, and Bowman’s capsule and smudgy mesangial staining for IgG3 (anti-IgG3 immunofluorescence staining, original magnification x 200). g) Diffuse punctate to amorphous electron dense deposits along tubular basement membranes, (transmission electron microscopy x 5000).
On electron microscopy (EM), the GBM lamina densa had normal thickness. Scattered small to medium amorphous and punctate deposits were present along the subendothelial GBM with approximately 60% podocyte foot process effacement, and no fibrin tactoids or tubuloreticular aggregates. Mesangial matrix was significantly increased, with moderate increase in cellularity, frequent amorphous mesangial deposits, and many tubular BM punctate and amorphous deposits.
Final diagnosis
IgG3 Heavy Chain Deposition Disease.
Clinical follow-up
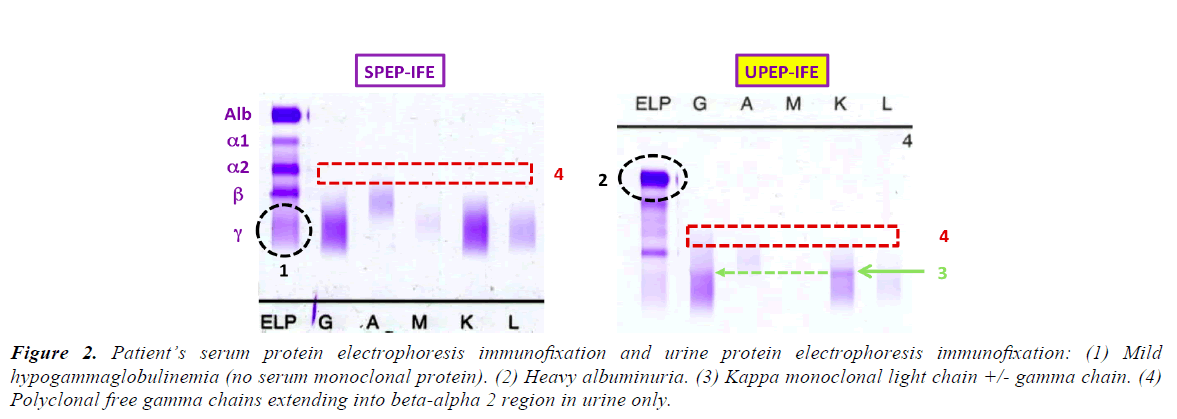
Although hepatitis C antibody was positive, hepatitis C PCR was undetectable. SPEP showed mild hypogammaglobulinemia with no serum monoclonal protein. UPEP showed albuminuria, a kappa band, likely a Bence Jones protein, and increased gamma staining. Serum free kappa light chains resulted 408.43 mg/L, free lambda 14.02 mg/L, ratio of kappa to lambda of 29.13.
Bone marrow biopsy demonstrated normocellular marrow (50-60% cellularity), 5% plasma cell neoplasm, which exhibit monotypic kappa light chain restriction by flow cytometry, reported as compatible with a plasma cell neoplasm. There was no overt expression of heavy chains. Skeletal survey was negative for lytic lesions. The diagnosis of Monoclonal Gammopathy of Undetermined Significance (MGUS) could not be confirmed due to the absence of M protein on SPEP. This patient met only two of the three criteria required for the diagnosis of MGUS, i.e., the presence of <10% plasma cells on bone marrow biopsy, and absence of lytic bone lesions. Treatment was aimed at the underlying plasma cell dyscrasia. The patient began dexamethasone and bortezomib, based on anecdotal evidence [3,6]. SCr improved to 1.0 mg/dl at 18 months, but anemia persisted out of proportion to renal dysfunction. A second bone marrow biopsy 11 months after initial hospitalization, showed no abnormal cell proliferation.
While hospitalized, the patient developed another episode of angioedema, unresponsive to solumedrol and antihistamines. C1q level was low at 7.2 mg/dL, suggesting Acquired C1 Inhibitor Deficiency. C1 inhibitor level was normal at 30 mg/ dl, and Cl functional level was lost on send out. The patient began aminocaproic acid, with no further angioedema during the hospital stay.
Discussion
Table 2 shows the differential diagnosis for nephritic syndrome associated with hypocomplementemia. The isolated positive UPEP with negative SPEP pattern is peculiar, and not consistent with known intact immunoglobulin diseases, including Membranoproliferative Glomerulonephritis (MPGN), Immunotactoid Glomerulonephritits, and Fibrillary Disease (Figure 2; Table 3) [7-12].
Figure 2. Patient’s serum protein electrophoresis immunofixation and urine protein electrophoresis immunofixation: (1) Mild hypogammaglobulinemia (no serum monoclonal protein). (2) Heavy albuminuria. (3) Kappa monoclonal light chain +/- gamma chain. (4) Polyclonal free gamma chains extending into beta-alpha 2 region in urine only.
| Nephritic syndromes with and without hypocomplimentenemia | ||
|---|---|---|
| Renal pathology without systemic involvement | Systemic Disease | |
| Low complement | Idiopathic Membranoproliferative Glomerulonephritis C3 Glomerulopathy (C3 low, C4 usually normal) | Lupus Nephritis Infectious GN Post-streptococcal GN Cryoglobulinemic/ HCV MPGN Immunotactoid GN Heavy Chain Deposition Disease |
| Normal complement | IgA Nephropathy Anti-GBM |
ANCA Vasculitis Henoch Schonlein Purpura Good Pasture Disease |
Table 2. Differential diagnosis of progressive Nephritic syndrome: association between complement levels and renal versus systemic involvement.
| Intact Immunoglobulin Disease | UPEP Immunofixation +ve SPEP Immunofixation -ve |
Reference |
|---|---|---|
| Type I Cryoglobulinemia | 0/31 | Néel et al. [7] |
| Cryoglobulinemia & Monoclonal Ig MPGN | 1/35** | Sethi and Rajkumar [8] |
| Proliferative GN with Monoclonal Ig | 0/11 | Nasr et al. [9] |
| Monoclonal Ig MPGN | 0/8 | Guiard [10] |
| Immunotactoid GN | 0/10 | Nasr et al. [11] |
| Immunotactoid GN | 0/5 | Bridoux et al. [12] |
| Total | 1/100 | - |
**Case with negative immunofloresence on renal biopsy
Table 3. Testing the hypothesis: Disease processes associated with an intact immunoglobulin mostly do not present with a negative SPEP and positive UPEP. This is because intact immunoglobulins cannot be cleared sufficiently into the tubular lumen, and hence be excreted as urine with undetectable serum levels, even with glomerular “porosity”.
This pattern is unique to Light Chain Diseases (AL Amyloid and Light Chain Deposition Disease (LCDD) or Heavy Chain Disease (HCDD or AH Amyloid), but hypocomplementemia is only seen in the latter. Light chains do not activate complement, as the complement-binding region is on the heavy chain.
Our patient’s younger age and kappa clone favor HCDD over AH Amyloid. The heavy chain is produced with a light chain, both of which are free. However, only the heavy chain deposits are pathogenic. This contrasts with Light and Heavy Chain Deposition Disease (LHCDD), where truncated heavy and light chains associate with each other, and behave as intact immunoglobulin, with both SPEP and UPEP either positive or negative.
Monoclonal Immunoglobulin Deposition Disease (MIDD) classification is based on deposition of a component of monoclonal immunoglobulin in kidneys and extra-renal tissues. HCDD is the least common MIDD.
MIDDs are distinctly different from other deposition diseases such as amyloid, immunotactoid glomerulonephritis, and cryoglobulenemia. MIDDs have non-organized immunoglobulin deposits, and are congo red stain negative. HCDD demonstrates this non-organized pattern in its glomerular truncated heavy chain distribution [13].
MIDD includes LCDD and LHCDD, which are often clinically indistinguishable from HCDD, with immunofluorescence needed for diagnosis. MIDDs are linked with immunoproliferative disorders, but have also been reported in the absence of overt malignancy, often presenting with renal dysfunction.
HCDD pathogenesis includes clonal expansion of B cells producing abnormal, truncated heavy chains with a deletion including the CH1 (constant) domain, and often the heavy chain Variable Region (VH) [14]. CH1 deletions allow abnormal heavy chains to be secreted freely into circulation. VH deletion may allow rapid tissue deposition, leading to undetectable serum heavy chains [14].
HCDD often involves kidneys primarily, with hematologic involvement in about 25% of patients [13]. Other extra-renal, less common deposition sites include cardiac, skin, synovial, pancreas, striated muscles, liver and thyroid [13].
Key renal biopsy findings include extensive extracellular matrix deposition with nodular glomerulosclerosis. Light microscopy reveals renal tubular BM and glomerular deposits of eosinophilic, PAS positive material, often with interstitial fibrosis. Glomerular mesangial matrix expansion with nodular glomerlosclerosis may resemble diabetic glomerulosclerosis, but is different because of the relatively regular nodular distribution pattern, and the absence of arteriolar hyalinosis [13]. Arteries, arterioles and capillaries may contain PAS positive deposits next to BMs [13].
Immunofluorescence stains positive for a single type of heavy chain (alpha, gamma or meu) along the glomerular and tubular BMs, and sometimes in the nodular lesions. Complement components (particularly C1) may also be seen in a granular pattern along the BM [13].
Stains should include kappa and lambda light chains, as both are negative in HCDD.
EM shows punctate or amorphous electron dense deposits along the tubular and glomerular BMs, and within mesangial nodules.
The false positive hepatitis C antibody and hypocomplimentenimia in HCCD can be misleading. Lin et al. in a report of 34 cases of MIDD found 4 of 5 HCDD patients had a positive HCV antibody test, undetectable HCV by PCR, and no evidence of active hepatitis [13]. Abnormal truncated heavy chains may interfere with the HCV immunoassay to cause a false-positive HCV antibody test [13].
Acquired angioedema often associates with lymphoproliferative disorders, resulting from a quantitative or functional inhibitor deficiency.1 Autoantibodies to the C1 inhibitor protease complex are hypothesized to cause C1 inhibitor cleavage or inactivation, resulting in decreased C1 inhibitor functional activity, low or normal C1 inhibitor levels, and low C1q levels [15-18]. In this case, pathogenic free heavy chains may have blocked C1 esterase inhibitor (C1-INH) function, predisposing to bradykinin mediated angioedema.
Binding of C1 to the Fc portion of IgM or IgG activates the classic component pathway. C1 has 3 subunits, C1q being the initial binding subunit, triggering proteolytic cleavage of C1r and C1s. C1s then acts as a protease for C4 and C2. Uncontrolled activation of C1s leads to low C2 and C4 levels [16,17].
C1 inhibitor is a regulator protein which inhibits the catalytic subunits of the C1 complex (C1r and C1s) and kallikrein pathway [16,19]. Decreased activity of the C1 inhibitor complex results in uncontrolled activation of the classic complement and kallikrein pathway, and excess bradykinin [19].
Low levels of C2, C4 should raise suspicion for acquired angioedema. Confirmatory tests include low C1q, decreased C1 inhibitor functional level, and low or normal C1 inhibitor level [16,17]. Several therapies including steroids, epinephrine, antihistamines, aminocaproic acid have been tried, none have proven effective and treatment should be aimed at the underlying disease.
Although HCDD is uncommon, it must be suspected in patients presenting with nephritic syndrome, hypocomplementenemia, and monoclonal immunoglobulin components in urine electrophoresis, possibly with negative serum electrophoresis. It is important to remember that the hepatitis antibody test may be falsely positive [14]. Current treatments include high dose steroids, bortezomib and autologous stem cell transplant [3,20]. Although prognosis remains uncertain, some cases demonstrate a response to these therapies with preserved renal function over several years.
References
- Katz A, Zent R, Bargman JM. IgG heavy-chain deposition disease. Mod Pathol. 1994;7(8):874-8.
- Kambham N, Markoqitz GS, Appel GB, et al. Heavy chain deposition disease: The disease spectrum. Am J Kidney Dis. 1999;33(5):954-62.
- Oe Y, Nakaya I, Yahata M, et al. A case of γ1-heavy chain deposition disease successfully treated with melphalan and prednisolone therapy. Intern Med. 2010;49(14):1411-5.
- Alexander MP, Nasr SH, Watson DC, et al. Renal crescentic alpha heavy chain deposition disease: a report of 3 cases and review of the literature. Am J Kidney Dis. 2011;58(4):621-5.
- Chen GT, Liao XH, Yan RY, et al. A case of renal γ2 heavy-chain deposition disease accompanied by rapid progressive renal failure. Ir J Med Sci. 2014;183(2):319-21.
- Ronco P, Plaisier E, Mougenot B, et al. Immunoglobulin light (heavy)-chain deposition disease: from molecular medicine to pathophysiology-driven therapy. Clin J Am Soc Nephrol. 2006;(1):1342-50.
- Néel A, Perrin F, Decaux O, et al. Long-term outcome of monoclonal (type 1) cryoglobulinemia. Am J Hematol. 2014;89:156-61.
- Sethi S, Rajkumar SV. Monoclonal Gammopathy-Associated Proliferative Glomerulonephritis. Clin J Am Soc Nephrol. 2010;5: 770-82.
- Nasr SH, Satoskar A, Markowitz GS. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20:2055-64.
- Guiard E, Karras A, Plaisier E, et al. Patterns of non-cryoglobulinemic glomerulonephritis with monoclonal Ig deposits: correlation with IgG subclass and response to rituximab. Clin J Am Soc Nephrol. 2011;6:1609-16.
- Nasr SH, Fidler ME, Cornell LD. Immunotactoid glomerulopathy: clinicopathologic and proteomic study. Nephrol Dial Transplant. 2012;27:4137-46.
- Bridoux F, Hugue V, Coldefy O, et al. Fibrillary glomerulonephritis and immunotactoid (microtubular) glomerulopathy are associated with distinct immunologic features. Kidney Int. 2002;62:1764-75.
- Lin J, Markowitz GS, Valeri AM, et al. Renal monoclonal immunoglobulin deposition disease: the disease spectrum. J Am Soc Nephrol. 2001;12(7):1482-92.
- Moulin B, Deret S, Mariette X, et al. Nodular glomerulosclerosis with deposition of monoclonal immunoglobulin heavy chains lacking CH1. J Am Soc Nephrol. 1999;(10):519-28.
- Patel K, Dillon JJ, Leung N, et al. Use of bortezomib in heavy-chain deposition disease: a report of 3 cases. Am J Kidney Dis. 2014;64(1):123-7.
- Markovic SN, Inwards DJ, Frigas EA, et al. Acquired C1 esterase Inhibitor Deficiency. Ann Intern Med. 2000;132(2):144-50.
- Shiozawa S, Shiozawa K. Angioedema and Acquired C1 esterase Inhibitor Deficiency. Editorial Intern Med. 2002:41(5):333-4.
- Cugno M, Castelli R, Cicardi M. Angioedema due to acquired C1-inhibitor deficiency: A bridging condition between autoimmunity and lymphoproliferation. Autoimmun Rev. 2008;8:156-9.
- Cicardi M, Johnston DT. Hereditary and Acquired Complement Component 1 Esterase Inhibitor Deficiency: A Review for Hematologist. Acta Haematol. 2012;127(4):208-20.
- Royer B, Arnulf B, Martinez F, et al. High dose chemotherapy in light chain or light and heavy chain deposition disease. Kidney Int. 2004;65(2):642-8.