Research Article - Journal of RNA and Genomics (2016) Volume 12, Issue 1
Tomato leaf curl New Delhi virus (ToLCNDV) encoded AC2 associates with host mirnas by directly interacting with AGO1
Vikash Kumar1,2*, and Afsar R Naqvi1,31Plant Molecular Biology, International Centre for Genetic Engineering and Biotechnology, Aruna Asaf Ali Marg, New Delhi-110067, India,
2Department of Physiology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee 53226, USA
3Department of Periodontics, University of Illinois at Chicago, Chicago, IL 60612, USA
- Corresponding Author:
- Vikash Kumar
Tel: +414-955-8296
Fax: vikashkumarhi@yahoo.com, vkumar@mcw.edu
E-mail: ombamalu@uwc.ac.za
Received Date: 01 July 2016; Revised Date: 31 August 2016; Accepted Date: 22 September 2016; Published Date: 26 September 2016
Copyright: © First Published by Allied Academies. This is an open access article, published under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0). This license permits non-commercial use, distribution and reproduction of the article, provided the original work is appropriately acknowledged with correct citation details
Abstract
We have previously shown the deregulation of various miRNAs post Tomato leaf curl New Delhi virus (ToLCNDV) infection. Host miRNAs can potentially bind to viral DNA and/or virus-encoded mRNAs thus acting as hosts’ antiviral defense. The goal of the present study was to investigate the mechanism of action of ToLCNDV encoded RNA silencing suppressor (RSS) AC2 on the host miRNA machinery. Here, we report the possible role of AC2 in miRNA dysregulation and found that AC2 associates with miR319 and miR172 which are deregulated during ToLCNDV infection. We furthermore confirmed this association was mediated by direct interaction of AC2 with AGO1 using in vitro and ex vivo assays. These results provide mechanistic insight on understanding of AC2-mediated deregulation of host miRNA pathway.
Keywords
ToLCNDV, AC2, AGO1, geminivirus, PTGS, miRNA
Introduction
Tomato leaf curl New Delhi virus (ToLCNDV; Family: Geminiviridae, Genus: Begomovirus) infects tomato and causes severe yield loss (~40% to 90%) (Saikia and Muniyappa, 1989). This family of viruses may contain monopartite (DNA-A) or bipartite (DNA-A and DNA-B) circular ssDNA genomes. The DNA-A component encodes six open reading frames (ORFs) namely AC1, AC2, AC3, AC4, AV1 and AV2 while, only two proteins BC1 and BV1 are encoded by DNA-B component (Dry et al, 1993; Padidam et al, 1995). AC2 encodes for a 15 kDa protein that functions as a pathogenicity factor and acts as an RSS (Voinnet et al, 1999; Kumar et al, 2014; Kumar et al, 2015).
MicroRNAs (miRNAs) are small (~22 nts), single stranded, non-coding RNA that regulate diverse biological processes, both in plants and animals (Bartel, 2004; Jones-Rhoades et al, 2006). Plant miRNAs are generated from pri-miRNA transcripts which form hairpin like structure and sequentially processed by the action of an RNA endoribonuclease, namely Dicer-like 1 (DCL1), working in a slicing complex comprising doublestranded RNA-binding protein HYPONASTIC LEAVES1 (HYL1), C2H2-zinc finger protein SERRATE (SE), and nuclear cap-binding complex (CBC) (Dong et al, 2008; Laubinger et al, 2008). The miRNA duplexes are transported into the cytoplasm from the nucleus by exportin 5 ortholog HASTY. Guide strand of miRNA is retained with AGO1 associated microRNA-induced silencing complex (mi-RISC) (Park et al, 2005). This complex binds to target RNA with sequence complementary to the guide miRNA strand and targets these for degradation or translational repression (Baumberger and Baulcombe, 2005; Brodersen et al, 2008; Sanan-Mishra et al, 2009).
Recently, expression of host miRNAs as well as mRNAs in response to geminivirus infection are shown to be deregulated and contributed to viral pathogenesis (Naqvi et al, 2010; Naqvi et al, 2011; Pradhan et al, 2015). Interestingly, some of them can potentially bind to the viral genomes as well as their encoded ORFs in host?s antiviral defense (Naqvi et al, 2011). Geminiviruses encode RSS proteins that are capable of interfering both post transcriptional gene silencing (PTGS) and transcriptional gene silencing (TGS) (Buchmann et al, 2009; Kumar et al, 2015). Remarkably, association of AC2 protein with host miRNAs is not yet known. Here, we report that ToLCNDV-AC2 associates with miRNAs in ToLCNDV infected tomato leaf. Further studies demonstrated that this association is not attributed to the RNA binding property of AC2 rather than its direct interaction with AGO1, an integral component of mi- RISC. These results provide mechanistic aspect of ToLCNDVAC2 mediated suppression of host miRNA-mediated pathway.
Material And Methods
Cloning of AC2 and plasmid construct
To obtain ToLCNDV-AC2 plant agroinfiltration construct, AC2 amplicon (422 bp) was amplified from ToLCNDV-A (DQ629101.1) template using a pair of primers listed below. AC2 Fwd 5´- ATGGATTCATGCGACCTTCGTCACCCTC-3´, AC2 Rev 5´- TTAAGAGCTCTAAATACCCTTAAGAAACGACCC-3´. The cloning was done using BamHI in the forward primer and SacI in the reverse. The vector pBI121 (Clontech, Palo Alto) was digested with the same pair of restriction enzymes.
ToLCNDV infection in tomato leaves
Agroinfiltration of tomato leaves constructs containing dimer of ToLCNDV-A (ADQ629101.1) and ToLCNDV-B (DQ169057) genomes were used to obtain ToLCNDV infected tomato leaf (Naqvi et al, 2011).
Immuno-precipitation and dot blot analysis
Total equal amount of isolated protein from the healthy and infected leaves was incubated with anti-AC2 antibody for 3 h at 4°C. Equilibrated protein-A sepharose beads (GE Healthcare, UK) was added to the reaction mixture and incubated for 1hr at 4°C on rocker. Beads were washed three times with wash buffer (75 mM NaCl, 0.05% NP-40, 100 mM Tris-Cl (pH 8), protease inhibitor cocktail and DTT). The Western blot and RNA dot blot were performed separately with same immunoprecipitate. The immunoprecipitate was blotted onto membrane and hybridized with ?P32 labeled DNA oligoes overnight at 40°C. The images were developed by scanning phosphor-imager screen to TYPHOON scanner (GE Healthcare). The sequence of DNA oligoes to miRNAs: miR156-AACTGTCTTCTATCTCTCGTG, miR156a*-TGACAGAAGCATAGAGAGCAC, miR172-TCTTAGAACTACTACGACGTA, miR172a*-TACACCGTATTAGTTCTAAGT, miR319-GTTCCTGACTTCCCTCGACC, miR319*-CTCGAGGAAGTCAGGTGTGT.
Yeast two-hybrid study
The DNA fragment encoding full length At AGO1 gene was amplified by PCR from cDNA of Arabidopsis using gene specific primers listed below and cloned into TOPO-TA cloning vector (Invitrogen, Carlsbad, CA, USA). The full length At AGO1 was digested with HindIII and SalI enzymes and then cloned into pSGI vector linearized by HindIII and SalI enzymes. Furthermore, AGO1 was excised from the SalI and HindIII sites of pSGI-AGO1 and re-cloned in pGADC1 (for activation domain fusion) and/or pGBDC1 (for DNA binding domain fusion). Similarly, AC2 was cloned in pGADC1 and pGBDC1 using BamHI and SalI sites. Yeast two hybrid assay was performed as described previously (Malik et al, 2005).
At AGO1 For: 5´- ATG TCG ACA TGG TGA GAA AGA GAA GAA CGG A-3´
At AGO1 Rev: 5´- GAA GCT TAG ATC TTC AGC AGT AGA ACA TGA CAC GCT-3´
In vitro translation of AGO1 and immunoprecipitation of ToLCNDV-AC2 with AGO1
pSGI-AGO1 construct was used to translate [35S] methionine radiolabeled proteins using the TnT T7/SP6 Coupled Wheat Germ Extract System (Promega, USA). In vitro translated At AGO1 proteins was incubated with purified ToLCNDV-AC2 recombinant protein or with other control proteins in a binding buffer (10 mM Tris-Cl pH 7.5, 100 mM to 200 mM NaCl, 5 mM MgCl2 and 0.2% BSA) for 30 min at room temperature. Recombinant ToLCNDV-AC2 was obtained by cloning AC2 into BamHI and SalI sites of pMal-c2x (New England Biolabs (NEB), MA) and purified by affinity chromatography with amylose resin (NEB, MA). After incubation, anti AC2 was added and the reaction was kept for 30 min at room temperature (RT) followed by incubation with protein A-Sepharose 4B for 30 min at RT on a rocker. Following incubation, the protein A-Sepharose beads were washed thrice with wash buffer (10 mM Tris-Cl, pH 7.5 and 2 mM EDTA). The beads were suspended in SDS sample buffer containing Ã-ME and were resolved on a 12% SDS-PAGE. The gel was exposed to phosphorimaging screen and visualized using phosphorimager.
Results
ToLCNDV-AC2 acts as an RNA silencing suppressor and associated with miRNAs from infected leaves
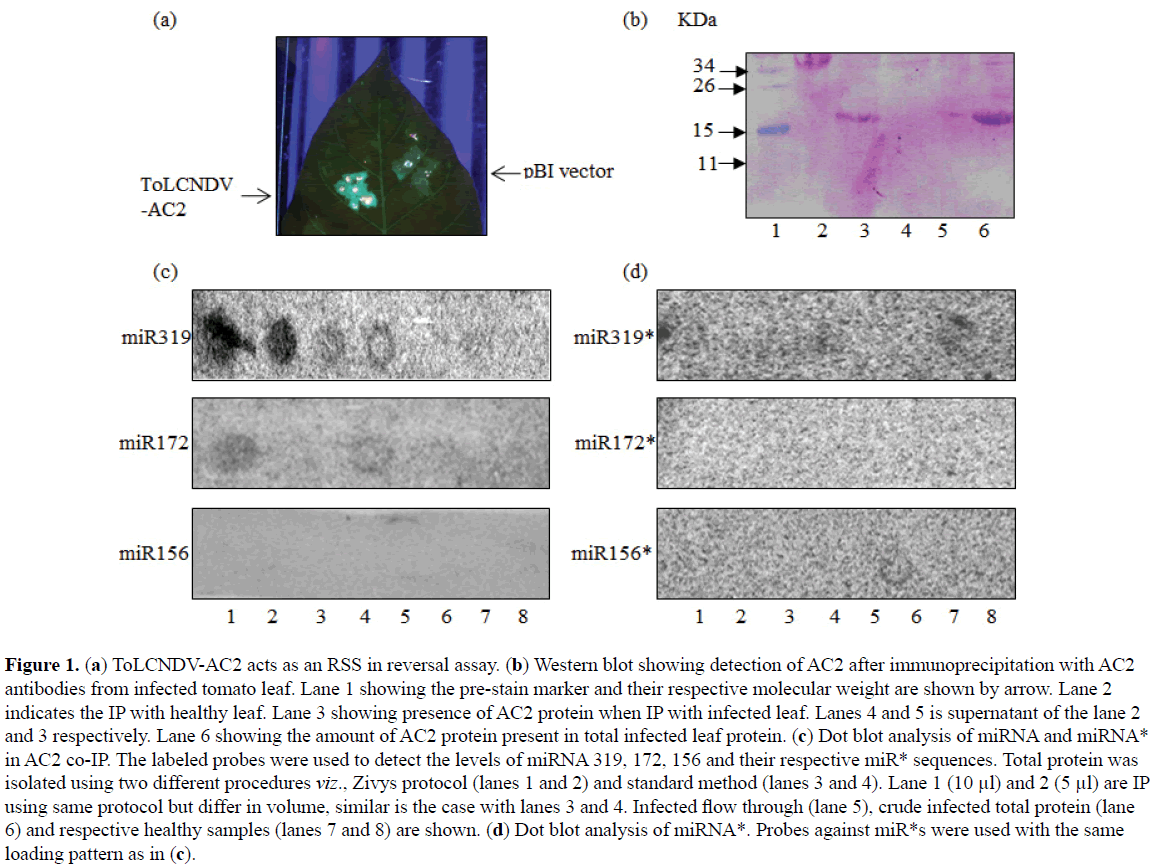
Geminiviral encoded AC2 has been shown to act as an RSS (Kumar et al, 2015). To test the ability of ToLCNDV encoded AC2 to suppress RNA silencing, AC2 was agroinfiltrated to GFP silenced N. tabaccum Xanthi transgenic plants. In principle, the GFP silenced plant which appears red under UV due to autofluorescence of chlorophyll reverts to the expression state in presence of the suppressor and appear fluorescent green under UV. The leaves of GFP silenced line agro-infiltrated with AC2 under 35S promoter reverted back the GFP expression 7 dpi under UV light (Figure 1A).
Figure 1: (a) ToLCNDV-AC2 acts as an RSS in reversal assay. (b) Western blot showing detection of AC2 after immunoprecipitation with AC2 antibodies from infected tomato leaf. Lane 1 showing the pre-stain marker and their respective molecular weight are shown by arrow. Lane 2 indicates the IP with healthy leaf. Lane 3 showing presence of AC2 protein when IP with infected leaf. Lanes 4 and 5 is supernatant of the lane 2 and 3 respectively. Lane 6 showing the amount of AC2 protein present in total infected leaf protein. (c) Dot blot analysis of miRNA and miRNA* in AC2 co-IP. The labeled probes were used to detect the levels of miRNA 319, 172, 156 and their respective miR* sequences. Total protein was isolated using two different procedures viz., Zivys protocol (lanes 1 and 2) and standard method (lanes 3 and 4). Lane 1 (10 µl) and 2 (5 µl) are IP using same protocol but differ in volume, similar is the case with lanes 3 and 4. Infected flow through (lane 5), crude infected total protein (lane 6) and respective healthy samples (lanes 7 and 8) are shown. (d) Dot blot analysis of miRNA*. Probes against miR*s were used with the same loading pattern as in (c).
Recently, our lab has demonstrated that ToLCNDV infection triggers the global changes of miRNA in the infected plant (Naqvi et al, 2010; Pradhan et al, 2015). Because miRs control several critical biological processes therefore it is reasonable to associate the role of altered miRs in the development and progression of ToLCNDV infection. Host miRNAs are considered as an antiviral response against viral genome as well transcripts. AC2 acts as an RSS which can potentially weaken the antiviral host defense. To determine whether miRNA associates with AC2 protein, immunoprecipitation (IP) of AC2 in ToLCNDV infected and healthy tomato leaf protein extract followed by dot blot was performed. Previously, we have shown that the level of miR319 and miR172 was elevated whereas miR156 did not exhibit variation among ToLCNDV infected and healthy tomato leaves (Naqvi et al, 2010). Thus we selected and probed these miRNAs in the dot-blot analysis. Figure 1B shows precipitation of AC2 only from ToLCNDV agroinfected leaves. Interestingly, miR319 and miR172 were detected in the co-IP, however, the level of miR156 was found to be below detection level (Figure 1C). Probes against miRNA* species did not exhibit any signal (Figure 1D). Taken together, our data suggest that ToLCNDV-AC2 acts as an RSS and associates with miRNAs.
Possible mechanism of RNA silencing suppression by ToLCNDV-AC2
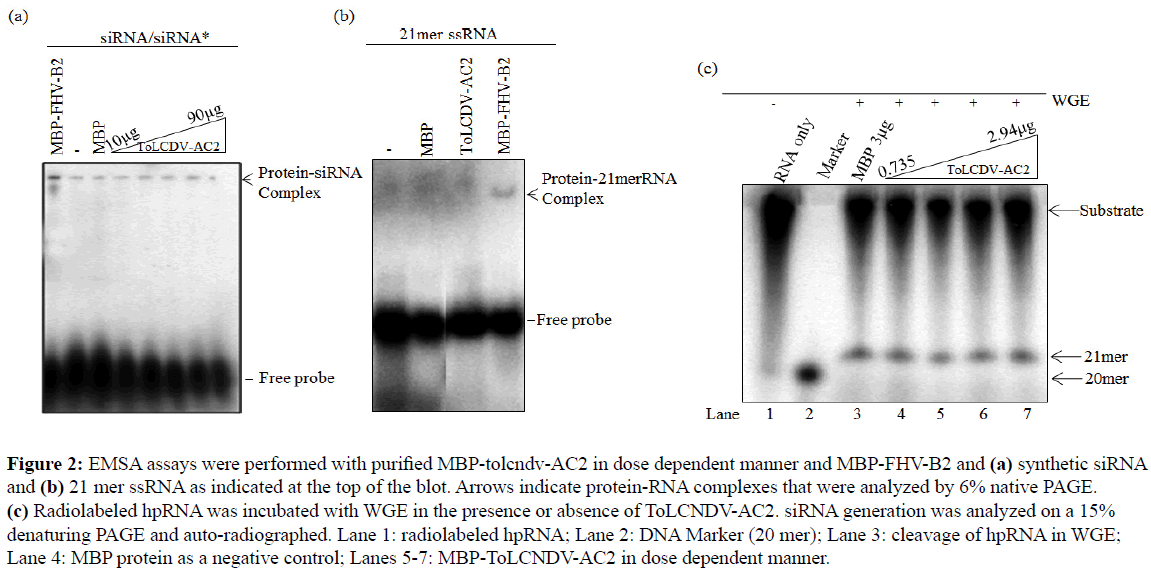
Geminiviruses encoded AC2 protein lacks RNA binding capability (Kumar et al, 2015), suggesting that either the ToLCNDV-AC2 protein might have an RNA binding activity or the observed association with miRNAs is mediated through an interaction with proteins that bind with miRNAs. To examine these possibilities, we carried out electrophoretic mobility shift assay (EMSA) for direct ds/ss siRNA binding activity of AC2. Radiolabeled siRNA molecules were incubated with MBPAC2 protein in a dose dependent manner (2 µg to 10 µg) and complexes were resolved by 6% native gel electrophoresis (Kumar et al, 2015). Our results show that MBP-AC2 did not bind to ds siRNA, however, a marked shift in siRNA was noticed when incubated with MBP-Flock horse Virus-B2, a known RSS that can form siRNA-protein complex (Figure 2A). Similar results were obtained when ss siRNA was used as a substrate (Figure 2B). Next, we investigated indirect mechanism for observed AC2-miRNA association, where we analyzed the interaction of AC2 with other protein components of RNA silencing pathway. We determined the effect of AC2 on the in vitro dicing activity using wheat germ extract (Kumar et al, 2015). The in vitro reaction was carried out at the concentration range of 0.735 µg to 2.94 µg but no observable difference was detected in the generation of ~21 nt siRNA from the radio-labeled hairpin dsRNA substrate (Figure 2C). Since no appreciable effect of AC2 on dicing activity was observed, we ruled out its involvement in the upstream miRNA or siRNA biogenesis pathway.
Figure 2: EMSA assays were performed with purified MBP-tolcndv-AC2 in dose dependent manner and MBP-FHV-B2 and (a) synthetic siRNA and (b) 21 mer ssRNA as indicated at the top of the blot. Arrows indicate protein-RNA complexes that were analyzed by 6% native PAGE. (c) Radiolabeled hpRNA was incubated with WGE in the presence or absence of ToLCNDV-AC2. siRNA generation was analyzed on a 15% denaturing PAGE and auto-radiographed. Lane 1: radiolabeled hpRNA; Lane 2: DNA Marker (20 mer); Lane 3: cleavage of hpRNA in WGE; Lane 4: MBP protein as a negative control; Lanes 5-7: MBP-ToLCNDV-AC2 in dose dependent manner.
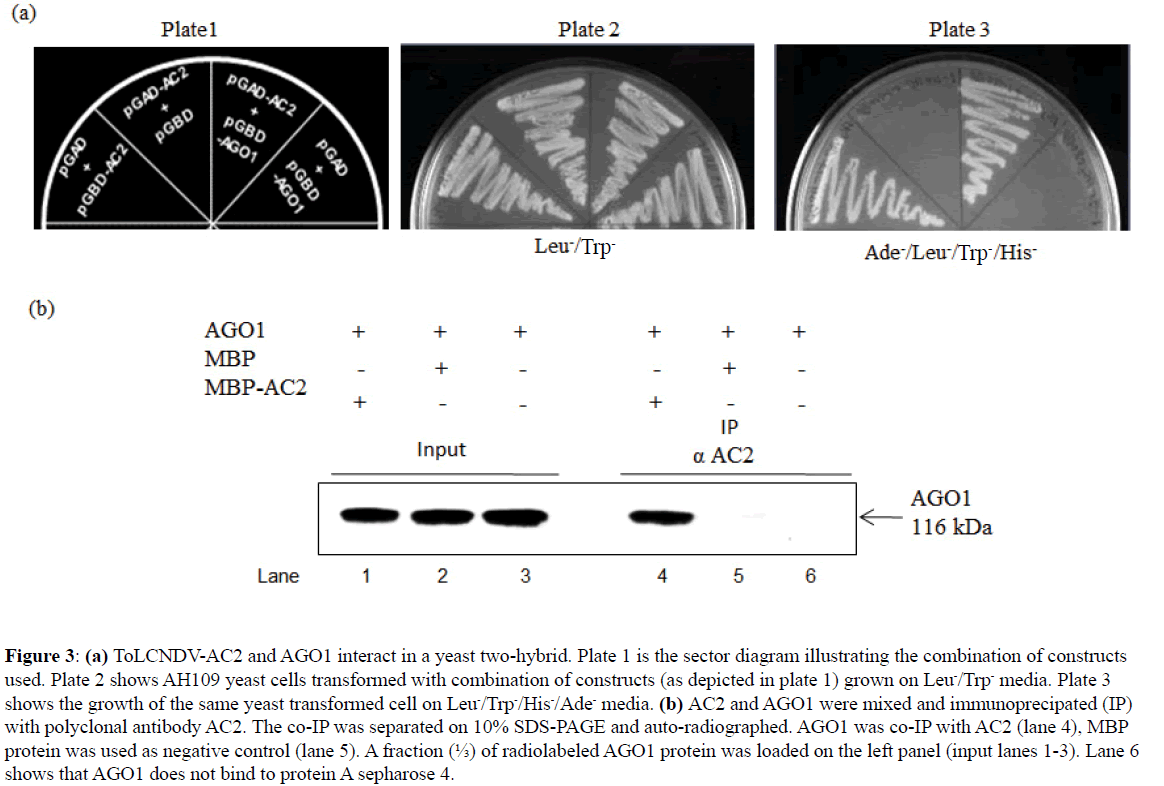
Another important protein of RNA silencing pathway, viz., AGO1 binds with miRNAs (ss and ds miRNA) for the RISC assembly, we therefore analyzed its interaction with AC2 ex vivo. We observed that yeast host strain harboring AC2 and AGO1 was able to grow on media lacking Ade-/His-/Trp-/Leuindicating that AC2 interacts with AGO1 (Figure 3A, Plate 3). This interaction was further substantiated by in vitro co-IP. Coimmunoprecipitation assay was carried out with radiolabeled AGO1 overproduced in wheat germ extract and incubated with recombinant MBP-AC2. Input was immunoprecipitated (IP) with polyclonal antibody to AC2 (antibody raised in rabbit against MBP-AC2). The autoradiogram revealed co-IP of AGO1 with the AC2 (Figure 3B), while no detectable band was observed with the tag alone (MBP) used as negative control (Figure 3B). Thus, the AC2-miRNA association we observed in the in planta immunoprecipitation is mediated by AC2-AGO1 interaction, where the latter associates with miRNAs for RISC assembly.
Figure 3: (a) ToLCNDV-AC2 and AGO1 interact in a yeast two-hybrid. Plate 1 is the sector diagram illustrating the combination of constructs used. Plate 2 shows AH109 yeast cells transformed with combination of constructs (as depicted in plate 1) grown on Leu-/Trp- media. Plate 3 shows the growth of the same yeast transformed cell on Leu-/Trp-/His-/Ade- media. (b) AC2 and AGO1 were mixed and immunoprecipated (IP) with polyclonal antibody AC2. The co-IP was separated on 10% SDS-PAGE and auto-radiographed. AGO1 was co-IP with AC2 (lane 4), MBP protein was used as negative control (lane 5). A fraction (?) of radiolabeled AGO1 protein was loaded on the left panel (input lanes 1-3). Lane 6 shows that AGO1 does not bind to protein A sepharose 4.
Discussion
Majority of the viral RSS binds to both siRNA and miRNAs. Interestingly, geminiviral encoded AC2 does not bind to any forms of RNA (Lakatos et al, 2006; Kumar et al, 2015). Our data further reconfirmed that ToLCNDV-AC2 also does not have binding ability to siRNAs. This finding is in agreement with other geminiviral encoded AC2 which also does not bind with either si- or miRNA. Recently it has been shown that Mungbean yellow mosaic India virus encoded AC2 binds and inhibits the AGO1 slicing activity in vitro (Kumar et al, 2015). AGO1 is the integral protein of RISC in plants and in vitro studies show that ToLCNDV-AC2 interacts with purified At AGO1. Taken together it seems that interaction of AC2 and AGO1 is the common theme to suppress host defense system. Previously, African cassava mosaic virus encoded AC4 which is an RSS has been shown to sequester mature miRNAs (Chellappan et al, 2005). However, it has not been shown which protein mediates this interaction as mature miRNAs are not easily accessible once incorporated into RISC assembly.
It has been recently proposed that capsid protein (P38) of Turnip crinckle virus and P1 of sweet potato mild mottle virus interact with host AGO via two GW/WG motifs to associate with miRNA pathway (Azevedo et al, 2010). P38 of TCV interacts with AGO1 with its GW motifs located at N- and C- terminals. HIV Nef protein which has strikingly similar motif arrangement to P38 is also shown to interact with host AGO2 (Aqil et al, 2013). Polerovirus P0 and P1 RSS interact with AGO via F-box motifs (Zhang et al, 2006). Even though ToLCNDV-AC2 does not possess any such motif and yet display interaction with AGO1. This could be through another protein that is part of AGO1-miRNA complex or AC2 might possess some AGO1 binding motif not yet identified in mediating this interaction. Further study is required to explore these possibilities.
MiRNA profiling of ToLCNDV agroinfected plants show global changes in miRNA expression (Naqvi et al, 2010; Pradhan et al, 2015). Polerovirus encoded P0 deregulate the miRNA regulation by directly inhibiting the AGO1 activity. This miRNAs regulation is critical for plant organ development and innate responses (Zhang et al, 2006). Co-IP with ToLCNDVAC2 pulled down miR319 and miR172 in ToLCNDV infected lysate but not in the healthy controls. This result supports that ToLCNDV-AC2 and AGO1 interaction can possibly affect plant miRNAs regulation. Employing in silico approach, we and others have demonstrated that various host miRNAs have potential binding sites on most of the viral transcripts (Pérez- Quintero et al, 2010; Naqvi et al, 2011). Interfering with miRNA pathway is thus crucial for survival of virus and both AC2 and AC4 could potentially interfere with host miRNA pathway. It will be interesting to examine whether these two proteins affect mutually exclusive RISC assemblies.
Conclusions
ToLCNDV-AC2 acts as an RNA silencing suppressor and does not bind with ss/ds siRNAs. AC2 associates with miRNA loaded AGO1 protein in RISC. These results provide mechanistic aspect of ToLCNDV-AC2 mediated suppression of host RNAi.
Acknowledgements
We thank Dr. Dharmendra Kumar Singh for his critical suggestions during the preparation of the manuscript.Competing Interests
None
List Of Abbrevations
ToLCNDV: tomato leaf curl New Delhi virus PTGS: post transcriptional gene silencing TGS: transcriptional gene silencing RISC: RNA induced silencing complex RSS: RNA silencing suppressor TrAP: transcriptional activator protein NLS: nuclear localization signal EMSA: electrophoretic mobility shift assay
References
- Aqil M, Naqvi AR, Bano AS and Jameel S. 2013. The HIV-1 nef protein binds argonaute-2 and functions as a viral suppressor of rna interference. PLoS One, 8, e74472.
- Azevedo J, Garcia D, Pontier D, Ohnesorge S, Yu A, Garcia S, BraunL, Bergdoll M, Hakimi MA, Lagrange T and Voinnet O. 2010.Argonaute quenching and global changes in dicer homeostasis caused by a pathogen-encoded GW repeat rotein. Genes Dev. 24, 904?915.
- Bartel DP. 2004. Micrornas: genomics, biogenesis, mechanism, and function. Cell. 116, 281?297.
- Baumberger N and Baulcombe DC. 2005. Arabidopsis argonaute1 is an RNA slicer that selectively recruits micrornas and short interfering rnas. Proc Natl Acad Sci USA. 102, 11928?11933.
- Brodersen P, Sakvarelidze-achard L, Bruun-rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L and Voinnet O. 2008. Widespread translational inhibition by plant miRNAs and siRNAs. Science. 320,1185?1190.
- Buchmann RC, Asad S, Wolf JN, Mohannath G and Bisaro DM.2009. Geminivirus al2 and l2 proteins suppress ranscriptional gene silencing and cause genome-wide reductions in cytosine methylation.J Virol. 83, 5005?5013.
- Chellappan P, Vanitharani R and Fauquet CM. 2005. Microrna-binding viral protein interferes with arabidopsis development. Proc Nat Acad Sci USA. 102, 10381?10386.
- Dong Z, Han MH and Fedoroff N. 2008. The RNA-binding proteins hyl1 and se promote accurate in vitro processing of pri-mirna by dcl1. Proc Natl Acad Sci USA. 105, 9970?9975.
- Dry IB, Ridgen JE, Krake LR, Mullineaux PM and Rezaian MA. 1993. Nucleotide sequence and genome organization of tomato leaf curl geminivirus. J Gen Virol. 74, 147?151.
- Jones-Rhoades MW, Bartel DP and Bartel B. 2006. Micrornas and their regulatory roles in plants. Annu Rev Plant Biol. 57, 19?53.
- Kumar V, Anand A, Mukherjee SK and Sanan-Mishra N. 2014. Engineering viral suppressors of RNA silencing: requirement and applications. In Reddy DVR, Kumar PA, Kumar PL, Loebenstein G and Rao CK, eds. Genetically Engineered Crops in Developing Countries. Studium Press, Texas, USA.
- Kumar V, Mishra SK, Rahman J, Taneja J, Sundaresan G, Mishra NS and Mukherjee SK. 2015. Mungbean yellow mosaic Indian virus encoded ac2 protein suppresses RNA silencing by inhibiting arabidopsis rdr6 and ago1 activities. Virology. 486, 158?172.
- Lakatos L, Csorba T, Pantaleo V, Chapman EJ, Carrington JC, Liu YP, Dolja VV, Calvino LF, López-Moya JJ and Burgyán J. 2006. Small RNA binding is a common strategy to suppress RNA silencing by several viral suppressors. Embo J. 25, 2768?2780.
- Laubinger S, Sachsenberg T, Zeller G, Busch W, Lohmann j. U, Rätsch G and Weigel D. 2008. Dual roles of the nuclear cap-binding complex and serrate in pre-mRNA splicing and microRNA processing in arabidopsis thaliana. Proc Natl Acad Sci USA. 105, 8795?8800.
- Malik PS, kumar V, Bagewadi B and Mukherjee SK. 2005. Interaction between coat protein and replication initiation protein of mung bean yellow mosaic India virus might lead to control of viral DNA replication. Virology. 337, 273?283.
- Naqvi AR, Haq QM, Mukherjee SK. 2010. MicroRNA profiling of tomato leaf curl New Delhi virus (ToLCNDV) infected tomato leaves indicates that deregulation of mir159/319 and mir172 might be linked with leaf curl disease. Virol J. 7, 281.
- Naqvi AR, Choudhury NR, Mukherjee SK and Haq QM. 2011. In silico analysis reveals that several tomato microRNA/microRNA sequences exhibit propensity to bind to tomato leaf curl virus (ToLCV) associated genomes and most of their encoded open reading frames (ORFs). Plant Physiol Biochem. 4 9, 13?17.
- Padidam M, Beachy RN and Fauquet CM. 1995. Tomato leaf curl geminivirus from India has a bipartite genome and coat protein is not essential for infectivity. J Gen Virol. 76, 25?35.
- Park MY, Wu G, Gonzalez-Sulser A, Vaucheret H and Poethig RS. 2005. Nuclear processing and export of microRNAs in arabidopsis. Proc Natl Acad Sci USA. 102, 3691?3696.
- Pérez-Quintero AL, Neme R, Zapata A and López C. 2010. Plant microRNAs and their role in defense against viruses: a bioinformatics approach. BMC Plant Boil. 10, 138.
- Pradhan B, Naqvi AR, Saraf S, Mukherjee SK, Dey N. 2015. Prediction and characterization of tomato leaf curl New Delhi virus (ToLCNDV) responsive novel microRNAs in Solanum lycopersicum. Virus Res. 195, 183?195.
- Saikia AK and Muniyappa V. 1989. Epidemiology and control of tomato leaf curl virus in southern India. Trop Agric. 66, 350?354.
- Sanan-Mishra N, Kuma V, Sopory SK and Mukherjee SK. 2009. Cloning and validation of novel miRNA from basmati rice indicates cross talk between abiotic and biotic stresses. Mol Genet Genomics. 282, 463?474.
- Voinnet O, Pinto YM and Baulcombe DC. 1999. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc Natl Acad Sci USA. 96, 14147?14152.
- Zhang X, Yuan YR, Pei Y, Lin SS, Tuschl T, Patel DJ and Chua NH. 2006. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis argonaute1 cleavage activity to counter plant defense. ?Genes Dev. 20, 3255?3268.