
allied
academies
Page 23
July 05-06, 2019 | Paris, France
Pharmaceutics and Advanced Drug Delivery Systems
2
nd
International Conference and Exhibition on
Asian Journal of Biomedical and Pharmaceutical Sciences | ISSN:2249-622X | Volume 9
Comparative pharmacokinetic study of Ledipasvir after single dose using novel meth-
odology
Saad A Alkahtani
Najran University, KSA
Background:
Ledipasivir (LEDV) is a direct acting antiviral used
for treatment of hepatitis C, especially in GT4 infection via
termination of HCV proliferation inside the body and has the
advantage of dose reduction compared to the other traditional
antiviral agents. Dose adjustment is highly important for
improving the efficacy of therapy and decreasing both the side
effects andpatient health's care cost. Toobtain clinically trusted
data, we should use highly sensitive and selective bio-analytical
techniques, capable of using small sample volumes, with no
interferences from endogenous or exogenous compounds.
Aim of work:
Therefore, pharmacokinetic study of LEDV was
investigated using novel validated highly sensitive sensor
obtained in our laboratories and comparing the results with
the reported results obtained by using LC/MS/MS technique.
Method:
Six volunteers had fed prohibited for 12 h before
the study but the water was freely available. The blood
samples (3.0 mL) were collected from a forearm vein into
heparinized polyethene tubes at 0.00 (pre-dose), 0.5, 1.0, 1.5,
2, 2.5, 3, 3.5, 4, 6, 10, 13, 18, 24 h after oral administration of
Harvoni®400/90 mg tablets. The samples were immediately
centrifuged at 4000 rpm for 10 min. The plasma was stored at
−80ºC until analysis. The pharmacokinetic parameters for LEDV
were estimated using the validated moment analysis software.
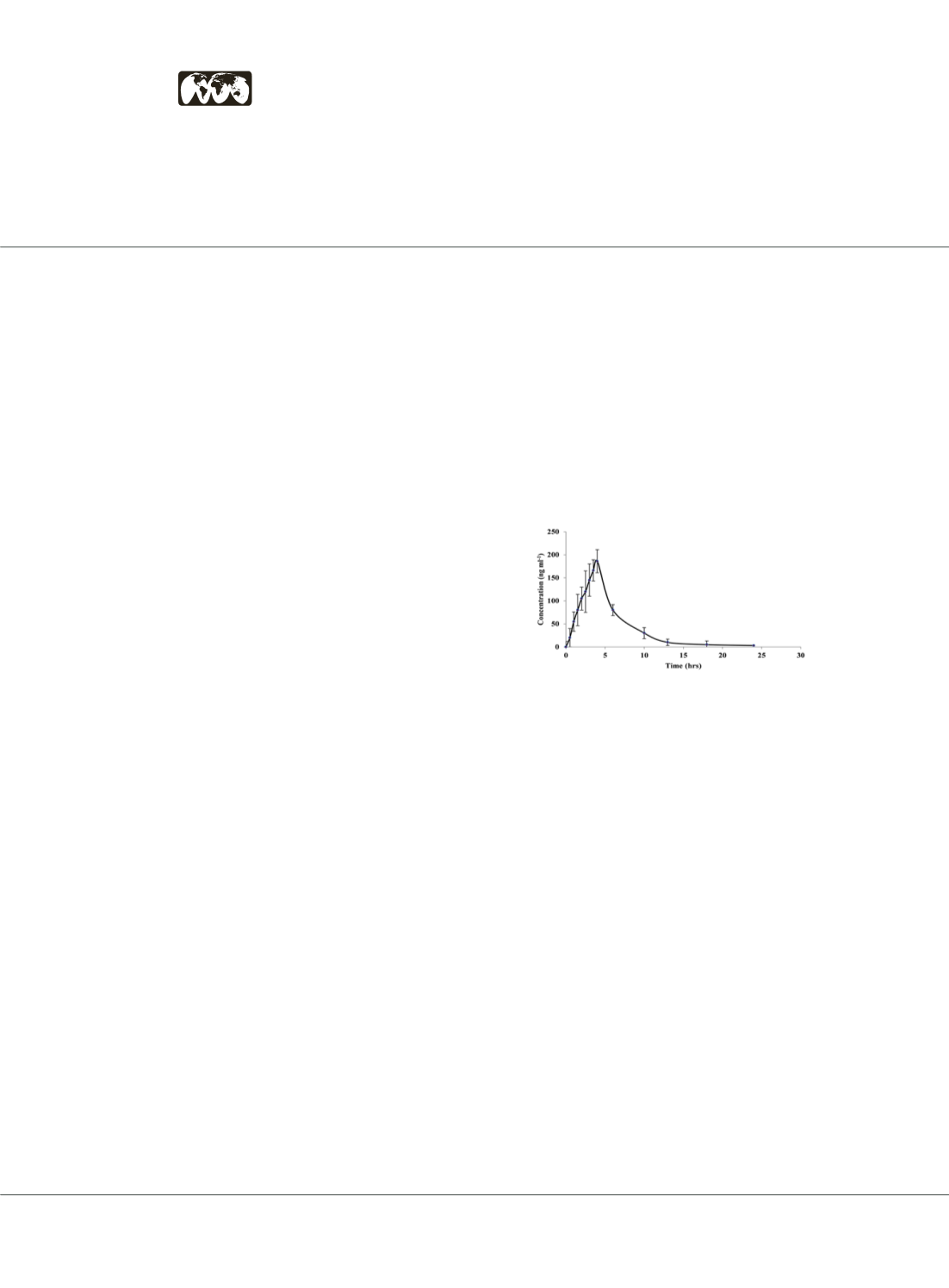
Results:
The methodology was fully validated according to
FDA guidelines with respect to linearity, accuracy, precision,
recovery, selectivity. The sensitivity of the method was
found to be sufficient for accurately measuring the main
pharmacokinetic parameters for LEDV. The validated
methodology was successfully applied to determine LEDV in
human plasma after oral administration of a tablet containing
400/90 mg SOF/LED. Following absorption, LEDV reaches
maximum plasma concentrations (T max) at 4.23± 2.09 h
post-dose and is eliminated with (t½) of 31.1± 2.6 h. The
Cmax was 183.7± 25.6 ng/mL, while AUC 0-t and AUC 0-∞
were 3709±1033 and 4201± 2345 ng/mL.h, respectively. The
elimination rate constant (Ke) and clearance (CL) were 0.026±
0.0001 h-1 and 0.034± 34.6 mg/(ng/mL h), respectively.
Our study proved that there was no significant difference
in pharmacokinetic parameters with other reported data.
Speaker Biography
Saad Alkahtani is currently Dean for College of Pharmacy at Najran
University, Saudi Arabia. He is also an Associate Professor of Clinical
Pharmacy, College of Pharmacy. Saad holds a PhD in Pediatric Clinical
Pharmacology from the University of Nottingham, UK, 2013. He earned
his Master's Degree from University of Glasgow, UK, 2009 and his
undergraduate studies at King Saud University, Saudi Arabia, 1999. His
research interest lies in evaluating cultural perceptions of, and access
to healthcare and pharmacy services. His other research interests
include pharmacoepidemiology and counterfeit medications. He has
collaborated actively with researchers in several other disciplines of
pharmaceutical sciences, particularly drug designing. He serves and
has served in various committees at the Faculty. He is and has been a
member of various national and international committees and working
groups in the area of clinical pharmacy and pharmacy education. He has
published many peer reviewed journal articles and conference papers
and he is a reviewer for several international peer-reviewed journals.
e:
saaalkahtani@nu.edu.saFigure 1. Mean plasma concentration of LEDV measured by RGO/ NS Ni Fe2O4/
MHS/GCE (±SD) using DPV at optimized conditions following administration of
single oral dose of Harvoni® tablets.
Saad Alkahtani
, Asian J Biomed Pharmaceut Sci, | ISSN: 2249-622X
Volume 9
















