Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2023) Volume 13, Issue 99
Sudden cardiac deaths before - after covid -19 pandemia and spike protein derivates uses
Mauro Luisetto1*, Almukhtar N2,Farhan Ahmad Khan3, Gamal A4, Coppolino Massimo5, Ansovini R6, Tarro G7, Cabianca L8, Mashori Gulam Rasool9, Fiazza C10, Yesvi Rafa A11, Nili B12, Oleg Yurevich Lathyshev13
11Marijnskaya academy , professorship general toxicology,applied and industrial chemistry branch italy
22Professor physiology, college medicine, university Babilon IRAQ.
33Department of Pharmacology, J.N. Medical College, AMU, Aligarh, India.
44Hamid Professor Hematology Oncology, University of Aden, Yemen.
5Molecular biologist and Professor of Genetic forensic italy.
6Medical researcher freelancer and inventor of Ansovini Technology, Italy.
7Professor of Oncologic virology,Chairman of the Committee on Biotechnologies of VirusSphere World Academy of Biomedical Technologies (WABT), Paris.
8Cabianca L medical laboratory turin italy Citta’ della Salute.
9Mashori Gulam Rasool, Professor, dr. Department of Medical & Health Sciences for Woman, institute of pharmaceutical science, Peoples University of Medical and Health Sciences for Women, Pakistan.
10Fiazza C , Medical pharmacologist, independent researcher Italy.
11Yesvi Rafa A, university of Nebraska -Lincoln NE,USA.
12Ahmadabadi ,Nano Drug Delivery, (a Product Development Firm), United States.
13Oleg Yurevich Lathyshev President IMA ACADEMY Marijnskaya RU.
- Corresponding Author:
- Mauro Luisetto
Marijnskaya academy
professorship general toxicology
applied and industrial chemistry branch
italy
E-mail: maurolu65@gmail.com
Received: 28-Apr-2023, Manuscript No. AABPS-23- 100065; Editor assigned: 01-May-2023, PreQC No. AABPS-23-100065(PQ); Reviewed: 15-May-2023, QC No. AABPS-23-10006; Revised: 19-May-2023, Manuscript No. AABPS-23- 100065(R); Published: 26-May-2023, DOI:10.35841/aabps-13.99.180
Citation: Luisetto M. Sudden cardiac deaths before - after covid -19 pandemia and spike protein derivates uses. Asian J Biomed Pharmaceut Sci. 2023;13(99):180
Keywords
Cardiac sudden deaths, Myocarditys, Pericarditis, SARS cov-2 spike protein derivates , Physiology epidemiology, Pathology, Toxicology.
Introduction
Great public debate is involved in the level of extra cardiac sudden deaths as reported in the media in last Periods. The same of interest to verify the movens of this phenomena and considering the fact that a severe pandemia ( covid-19 – S.A.R.S. cov-2 ) produced high number of deaths ( in acute way ) but also as long Covid-19 sequelae. To be considered on the global mortality rate also the effect played by the protocols adopted ; preventive measure ( lockdown and other ) or therapeutic option ( drug used or not used ): interesting literature report the objective analisys in neutral scientific way and the entity of the effect. The history of this time under eveluation reported various research published or changing the protocols to be used : see the hydroxiclorochin case. Various effect was due to “ Pandemia inside the pandemia” .The pathologic effect for the heart and other organ of the virus is due by the SPIKE protein toxicity and by the great immune reponce of the body to fight this micro organism. The toxicity of the spike protein is clearly showed by scientifi literature without no doubt.
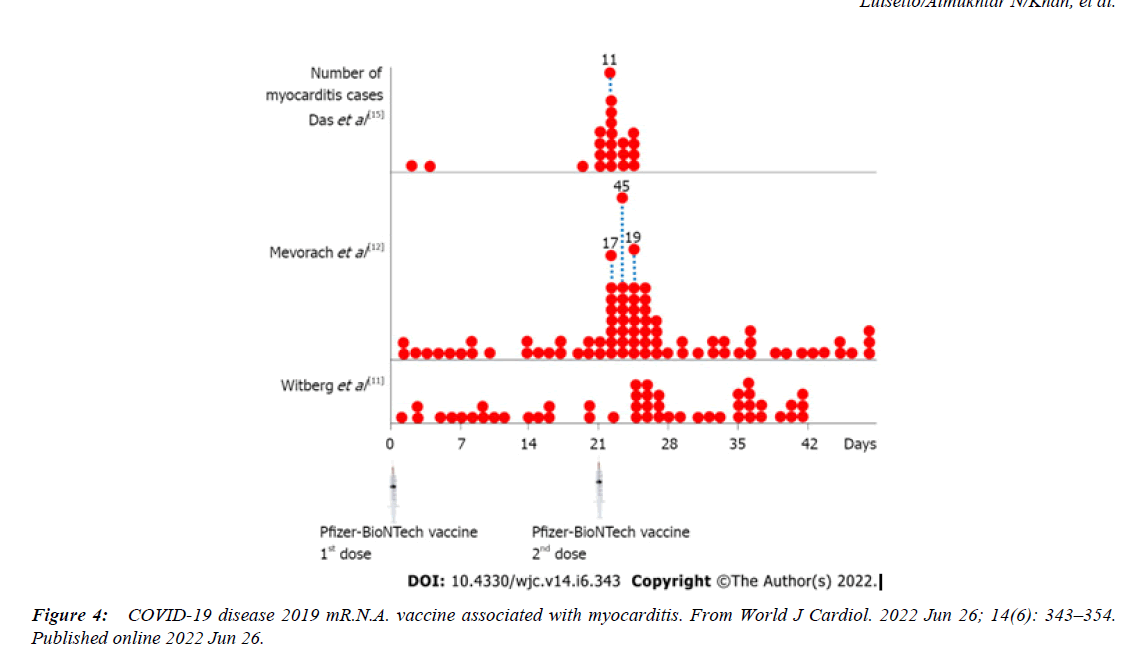
The fact that some covid-19 vaccine use Spike protein derivates ( or system to produce inside the body this product like mRna vaccine ) to stimualte immune responce generate the need to verify its profisle of safety also related cardiac sudden deaths and not only for other risks ( central nervous system trombosys et other ). For this reason it is usefull to observe epidemiology of sudden deaths in year before pandemia and to compare with pandemic season , and verifying also the effect played dy long covid syndrome and if possible to divide the effect played by the vaccination campaign. All this factor can be considered as cofactor? And there is an summatory of pathological effect ? The Rare ADR registered in some m RNA vaccine like miocarditys – pericarditys in youngs and the fact that some authorities restricted use of some product to determinate class age are facts that need to better study this phenomena . This work start observig what reported in the recente article International Journal of Advanced Research in Science, Communication and Technology (IJARSCT) Electronic Devices (WCR) and Covid-19 Vaccine ADR: Myocarditys and Pericarditis -Epidemiology and Physiology of An Interesting Phenomena after observing this evidence published in scientific article and observing that the mediana ( age ) of RARE miocardits after covid-19 vaccine seen differ form the classic myocarditys ( more frequent in young in vaccinated ) and related the profile of the covid-19 disease ( higer risk of miocarditis > 50 year ) and the role played by comorbidities it is of interest to match the geographic distribution of miocarditis after covid-19 vaccine with the use of WCR device .This because this 2 factor ( Spike protein natural or artificial ) and WCR radiation are recognized able to Influence heart as reported in literature.
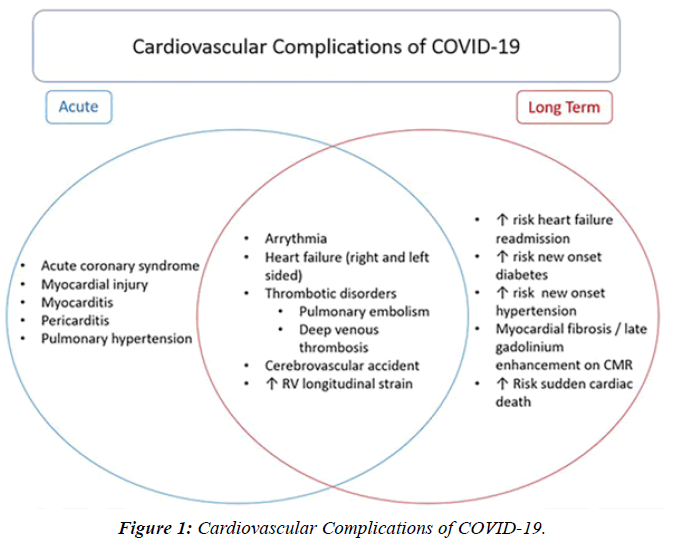
“Compared to uninfected individuals, the risk of death in COVID-19 patients was up to 81-fold higher in the acute phase and 5-fold higher in the post-acute phase. Patients with severe COVID-19 were more likely to develop major CV disease or die than non-severe cases”. The results showed that the adjusted incident rate ratios of CV outcomes in the post-COVID-19 exposure period were significantly higher than those in the pre-exposure period (ratios of incident rate ratios for all CV outcomes were significantly higher than 1) and exhibited a graded increase by severity of the acute phase of the disease . Cardiac arrest (HR = 2.45 (2.08, 2.89)” Myocarditis is a condition caused by acute or chronic inflammation of the cardiac myocytes, resulting in associated myocardial edema and myocardial injury - necrosis. The exact incidence is unknown, but is likely underestimated, with more mild cases going unreported. Diagnosis and appropriate management are paramount in pediatric myocarditis, as it remains a recognized cause of sudden cardiac death SCD in children and athletes. Myocarditis in children is most often caused by a viral or infectious etiology. There are now 2 highly recognized etiologies related to COVID-19 infection and the COVID-19 mR.N.A. vaccine. Autopsy-based histopathological characterization of myocarditis after anti- S.A.R.S.-CoV-2-vaccination.
Constantin Schwab, Lisa Maria Domke, Laura Hartmann, A. Stenzinger, Thomas Longerich & Peter Schirmacher. In general, a causal link between myocarditis and anti-S.A.R.S.- CoV-2 vaccination is supported by several considerations: - a close temporal relation to vaccination; all cases were found dead within 1 week after vaccination, -absence of any other significant pre-existing heart disease, especially ischaemic heart disease or cardiomyopathy, - negative testing for potential myocarditis-causing infectious agents, - presence of a peculiar CD4 predominant T-cell infiltrate, suggesting an immune mediated mechanism. The latter criterion is supported by demonstration of a phenotypically identical T-cell infiltrate at the deltoidal injection site in 1 of the cases. According Covid-19: Pandemic disruption linked to 30 000 excess heart disease deaths, charity reports.
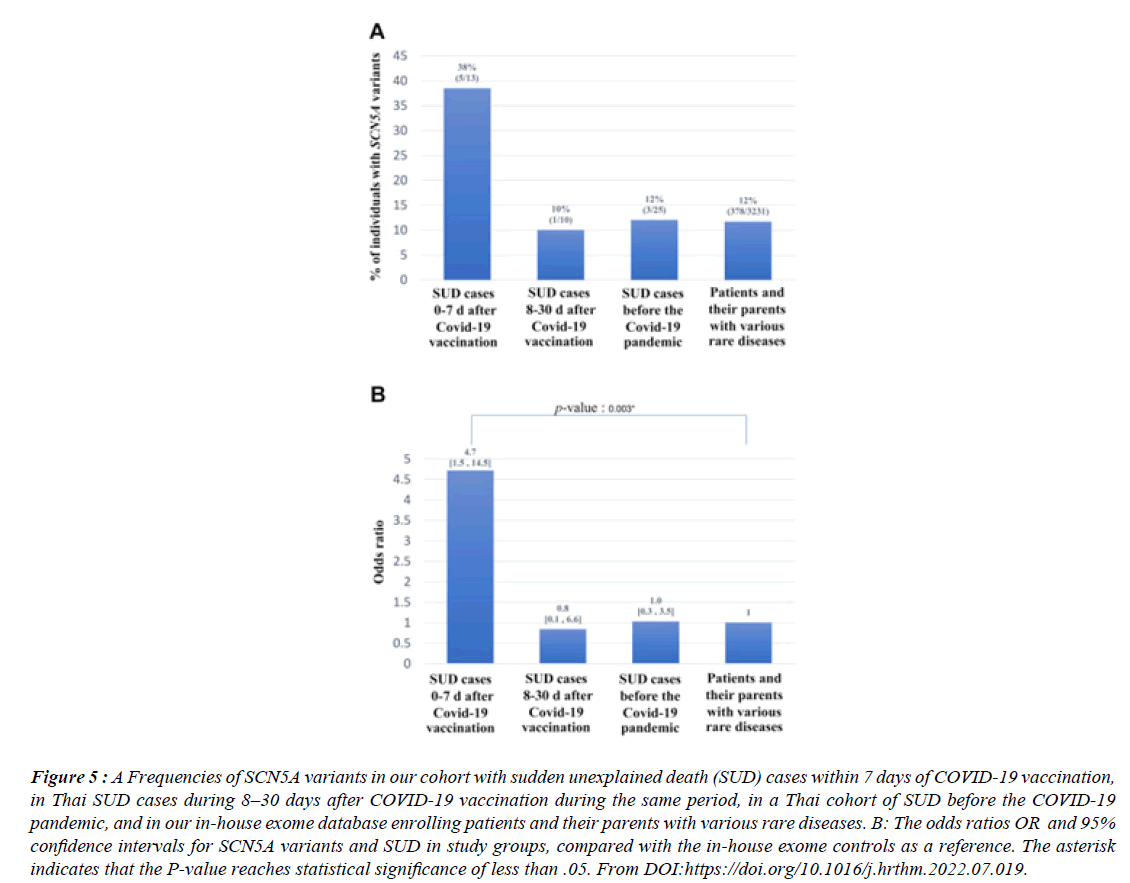
Between March 2020 and Aug 2022 there were more than 30 000 excess deaths involving coronary heart disease, an average of 230 a week above the expected death rate. Many people have not been able to access care for conditions like as high blood pressure, that could raise the risk of a future heart attack or stroke, the foundation’s report said. More than 650,000 deaths were registered in the UK in 2022 - 9% more than 2019. “S.A.R.S. COV-2 vaccination reduces morbidity and mortality associated with covid-19 disease ; unfortunately, it is associated with serious adverse events AE, including sudden unexplained death (SUD). 13 were recruited, aged between 23 and 72 years; 10 (77%) were men, 12 were Thai; and 1 was Australian. Eight (61%) died after receiving the first dose of vaccine, and 7 (54%) died after receiving ChAdOx1 nCoV-19.
There were no significant correlations between SUD and either the number or the type of vaccine. Fever was selfreported in 3 cases. Ten (77%) and 11 (85%) died within 24 hours and 3 days of vaccination, respectively. Whole exome sequencing analysis revealed that 5 cases harbored SCN5A variants that had previously been identified in patients with Brugada syndrome BS , giving an SCN5A variant frequency of 38% (5 of 13). This is a significantly higher rate than that observed in Thai SUD cases occurring 8–30 days after COVID-19 vaccination during the same period (10% [1 of 10]), in a Thai SUD cohort studied before the COVID-19 pandemic (12% [3 of 25]), and in our in-house exome database (12% [386 of 3231]).” Brugada (brew-GAH-dah) syndrome is a rare but potentially life-threatening heart rhythm condition (arrhythmia) that is sometimes inherited.
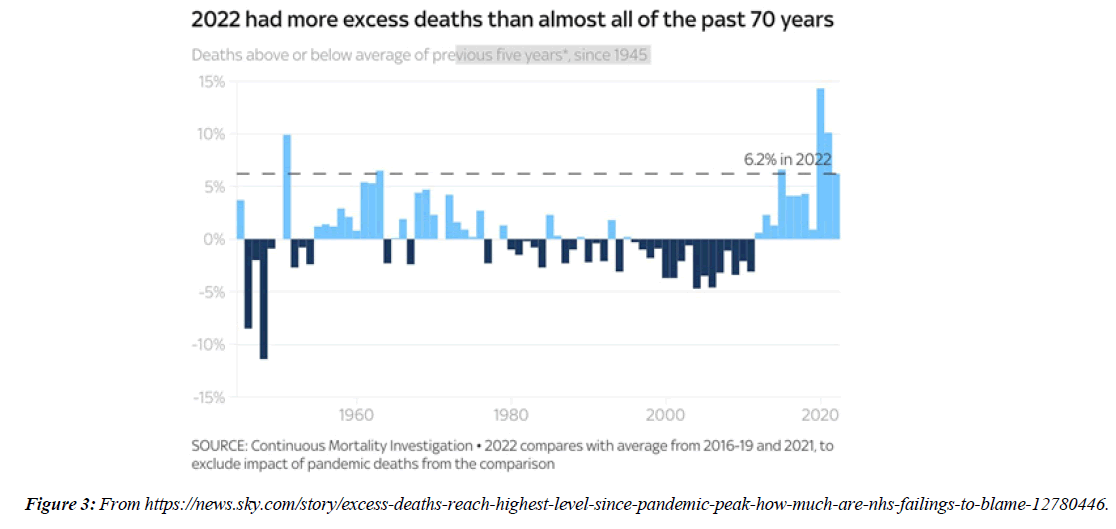
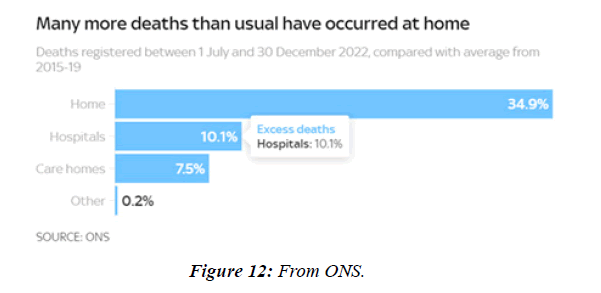
People with Brugada syndrome have an increased risk of irregular heart rhythms beginning in the lower chambers of the heart (ventricles) CV adverse outcomes such as blood clotting ( coronary artery thrombosis), acute coronary syndrome, cardiac arrest and myocarditis have been identified as consequences of COVID-19 infection. Data from regulatory surveillance and self-reporting systems, including the Vaccine Adverse events Reporting System in the US, the Yellow Card System in the U. K. and the EudraVigilance system in Europe, associate similar CV side-effects with a number of COVID-19 vaccines currently in use. Since July 2021, 7 months after the vaccination programme started in earnest, over 10,000 more people than usual have died in England and Wales alone from conditions unrelated to coronavirus, according to data from the Office for National Statistics NS. Paul Hunter, professor in medicine at the Univ. of East Anglia, notes that :in general the excess deaths seem to be primarily CV , and that many of them are happening at home. 40 hospitals in Washington, Oregon, Montana, and L A County, California, that were part of the Providence health care system and used the same electronic medical record were included. All patients with documented COVID-19 vaccinations administered inside the system or recorded in state registries at any time through May 25, 2021, were identified. Vaccinated patients who subsequently had emergency department ED or inpatient encounters with diagnoses of myocarditis, myopericarditis, or pericarditis were ascertained from EMRs. Extra deaths in 2022 close to highest level in 70 years - how much are NHS failings to blame? “More deaths at homeMany of the recent excess deaths are occurring at home, as opposed to in hospitals or care homes. In the 2 weeks to 30 Dec, 1,804 more people died at home than usual, almost 40% higher than the 5-year average. In total over the past 6 months 84,337 people have died at home, 21,803 (34.9%) more than the 5-year average from 2015-19. That's a much bigger difference than the change in other settings.
Materials and methods
With an observational point of view some relevant literature and figure ( 1-23) are reported . An experimental hypotesys is submitted to the reseacher in order to provide a global conclusion related the topic of investigation. All literature comes from Pubmed or other relevant biomedical database.
Results
Myocarditis and sudden death SD after COVID-19 mR.N.A. vaccination. Myocarditis induced by S.A.R.S.-CoV-2 mR.N.A. vaccines is an indisputable complication observed particularly in young males as demonstrated by multiple studies in different populations. 2 studies reported that COVID-19 mR.N.A. vaccine-induced myocarditis disproportionately affected adolescents (reporting odds ratio (ROR): 22.3; 95% confidence interval (CI): 19.2-25.9), 18-29-year-olds (ROR: 6.6; 95% CI: 5.9-7.5), and males (ROR: 9.4; 95% CI: 8.3- 10.6).
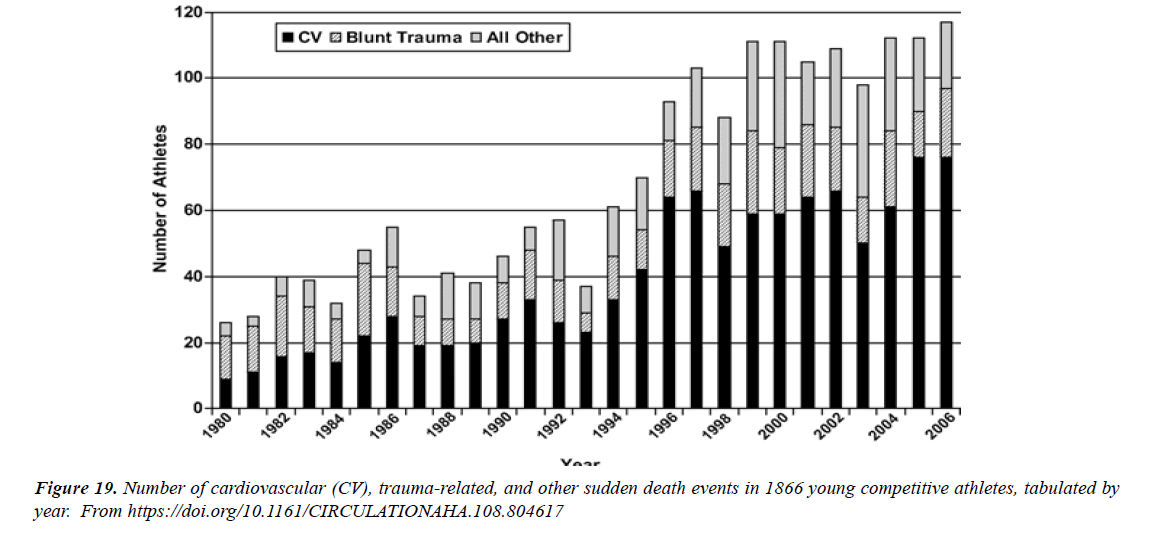
These findings were supported by other great registry study that identified increased myocarditis risk MR following S.A.R.S.-CoV-2 mR.N.A. vaccination, with the highest risk detected in people aged 18-24 years, particularly after 2nd dose, where 8.1-fold increased risk after the BNT162b2 S.A.R.S.-CoV-2 mR.N.A. vaccine (95% CI: 6.7-9.9) and 30- fold increased risk after the mR.N.A.-1273 S.A.R.S.-CoV-2 vaccine (95% CI: 21-43) were reported . An Israeli government dataset demonstrated 13.6-fold increased myocarditis risk (95% CI: 9.3-19.2) among males aged between 16 - 19 years compared to the expected following historical data, while a ninefold increased myocarditis risk (95% CI: 4.5-17.8) was recorded when compared to unvaccinated of similar age and sex during the same period . In a 23 million-resident area, the myocarditis risk MR after S.A.R.S.-CoV-2 mR.N.A. vaccination was increased across all populations , particularly high among males aged 16-24 years after the 2nd dose of BNT162b2 and mR.N.A.-1273, where 5.3-fold (95% CI: 3.7- 7.7) and 13.8-fold (95% CI: 8.1-23.7) increased myocarditis risk MR was recorded .Independent autopsies or biopsies of patients who presented post-S.A.R.S.- CoV-2 mR.N.A. vaccine myocarditis in different geographical regions enabled the conclusion that a primary hypercatecholaminergic state was the key trigger of these events; S.A.R.S.-CoV-2 mR.N.A. was densely present, and S.A.R.S.-CoV-2 spike protein was progressively produced in adrenal medulla chromaffin cells, which are responsible for catecholamine production; the dihydroxyphenylalanine decarboxylase enzyme that converts dopamine into noradrenaline was overexpressed in the presence of S.A.R.S.-CoV-2 mR.N.A., leading to enhanced noradrenaline activity; catecholamine responses were physiologically higher in young adults and males than in other populations; catecholamine responses and the resting catecholamine production were higher in male athletes than in non-athletes; catecholamine responses to stress and its sensitivity were enhanced in the presence of androgens; and catecholamine expressions in young male athletes were already high at baseline, were higher following vaccination, and were higher than those in non-vaccinated athletes.
The epidemiological, autopsy, molecular, and physiological findings unanimously and strongly suggest that a hypercatecholaminergic state is the critical trigger of the rare cases of myocarditis due to components from S.A.R.S.- CoV-2, potentially increasing sudden deaths SD among elite male athletes [1]. The demographic and clinical characteristics of myocarditis after mR.N.A. vaccination in previous reports are summarized in Table reported . Vaccine-associated myocarditis has been reported predominantly in young males after second vaccination. Myocarditis was mainly diagnosed clinically based on elevated serum troponin and cardiac magnetic resonance imaging, and all patients except ours and the patient reported by Verma et al. Recovered after receiving supportive care [2]. The incidence of sudden cardiac death ranged from 0.93 to 1.3 per 100 000 person-years , whereas an incidence of 1.62 per 100 000 person-years was reported in this research study [3].“The study examined 4 large ( >2 million inhabitants) European population-based prospective registries collecting emergency medical services –attended (ie, with attempted resuscitation) OHCA and SCD (OHCA without obvious extracardiac causes) for >5 consecutive years from Jan 2012 to Dec 2017 in the Paris region (France), the North Holland region , the Stockholm region (Sweden), and in all of Denmark.The average annual incidence of SCD in the 4 registries ranged from 36.8 per 100,000 (95% CI: 23.5- 50.1 per 100,000) to 39.7 per 100,000 (95% CI: 32.6-46.8 per 100,000) [4].
Cardiovascular CV effects of COVID-19 vaccination. Myocarditis and pericarditis are known complications of mR.N.A. vaccines, especially in young adult and teen males aged 12–17 years,23 with the highest observed incidence within 2–7 days after the second dose at a rate of 3.5–140 per million doses [5]. Additional analyses of CDC Vaccine Safety Datalink with data from 9 participating integrated health care organizations revealed an increased risk of myocarditis/ pericarditis events among individuals 12 to 39 years of age in the 7-day risk interval after the vaccination with mR.N.A. COVID-19 vaccines compared with unvaccinated individuals or individuals vaccinated with non-mR.N.A. COVID-19 vaccines on the same calendar days (rate ratio of 10.8 [95% CI, 3.2–49.0], adjusted for site, age, sex, race/ethnicity, and calendar date). The estimated myocarditis/pericarditis chartconfirmed rate was 12.6 cases per million doses with seconddose.
mR.N.A. vaccine among individuals 12 to 39 years of age. The rates based on International Classification of Diseases, 10th Revision–coded cases were also higher in males than in the females. All chart-confirmed cases with follow-up had resolution of symptoms; and among those who had follow-up ECG/echocardiography and lab. testing, most had returned to normal or baseline. On this basis, the FDA will add a warning to the product label of both mR.N.A. vaccines regarding the risk of myocarditis [6] and according Wai Hong Wilson Tang.
“In general, treatment of either acute or chronic myocarditis is aimed at reducing congestion and improving cardiac hemodynamics in heart failure HF , as well as providing supportive therapy, with the hope of prolonging survival. Treatment of heart failure follows the same treatment regimen regardless of the underlying cause. ( ACE inhibitors, betaadrenergic blockers).”[7]
We present autopsy findings of a 22-year-old man who developed chest pain 5 days after the first dose of the BNT162b2 mR.N.A. vaccine and died 7 hours later. Histological examination of the heart revealed isolated atrial myocarditis, with neutrophil and histiocyte predominance. Immunohistochemical C4d staining revealed scattered singlecell necrosis of myocytes which was not accompanied by inflammatory infiltrates. Extensive contraction band necrosis was observed in the atria and ventricles. There was no evidence of microthrombosis or infection in the heart and other organs. The primary cause of death was determined to be myocarditis, causally-associated with the BNT162b2 vaccine [8].
This study focuses on CV manifestation, particularly myocarditis and pericarditis events, after BNT162b2 mR.N.A. COVID-19 vaccine injection in Thai adolescents. This prospective cohort study enrolled students aged 13-18 years from 2 schools, who received the second dose of the BNT162b2 mR.N.A. COVID-19 vaccine. Data including demographics, symptoms, vital signs, ECG, echocardiography, and cardiac enzymes were collected at baseline, Day 3, 7, and Day 14 (optional) using case record forms. We enrolled 314 participants; of these, 13 participants were lost to follow-up, leaving 301 participants for analysis. The most common CV signs and symptoms were tachycardia (7.64%), shortness of breath (6.64%), palpitation (4.32%), chest pain (4.32%), and hypertension (3.99%). One participant could have more than 1 sign and/or symptom. 7 participants (2.33%) exhibited at least 1 elevated cardiac biomarker or positive lab assessments. CV manifestations were found in 29.24% of patients, ranging from tachycardia or palpitation to myopericarditis. Myopericarditis was confirmed in 1 patient after vaccination. 2 patients had suspected pericarditis and 4 patients had suspected subclinical myocarditis. CV manifestation in adolescents after BNT162b2 mR.N.A. COVID-19 vaccination included tachycardia, palpitation, and myopericarditis. The clinical presentation of myopericarditis after vaccination was usually mild and temporary, with all cases fully recovering within 14 days. Adolescents receiving mR.N.A. vaccines should be monitored for CV side effects. Clinical Trial Registration: NCT05288231[9].
Cases of myocarditis, diagnosed clinically by laboratory tests and imaging have been described in the context of mR.N.A.- based anti-S.A.R.S.-CoV-2 vaccination. Autopsy-based description of detailed histological features of vaccine-induced myocarditis is lacking. We describe the autopsy findings and common characteristics of myocarditis in untreated persons who received anti-S.A.R.S.-CoV-2 vaccination. Standardized autopsies were performed on 25 persons who had died unexpectedly and within 20 days after anti-S.A.R.S.- CoV-2 vaccination. In 4 patients who received a mR.N.A. vaccination, we identified acute (epi-)myocarditis without detection of another significant disease or health constellation that may have caused an unexpected death. Histology showed patchy interstitial myocardial T-lymphocytic infiltration, predominantly of the CD4 positive subset, associated with mild myocyte damage. Autopsy findings indicated death due to acute arrhythmogenic cardiac failure CF . Myocarditis can be a potentially lethal complication following mR.N.A.- based anti-S.A.R.S.-CoV-2 vaccination. Our findings may aid in adequately diagnosing unclear cases after vaccination and in establishing a timely diagnosis in vivo, providing the framework for adequate monitoring and early treatment of severe clinical cases [10]. CV events occur more frequently in the early morning, with morning rates higher for both AMI and sudden cardiac death [11].
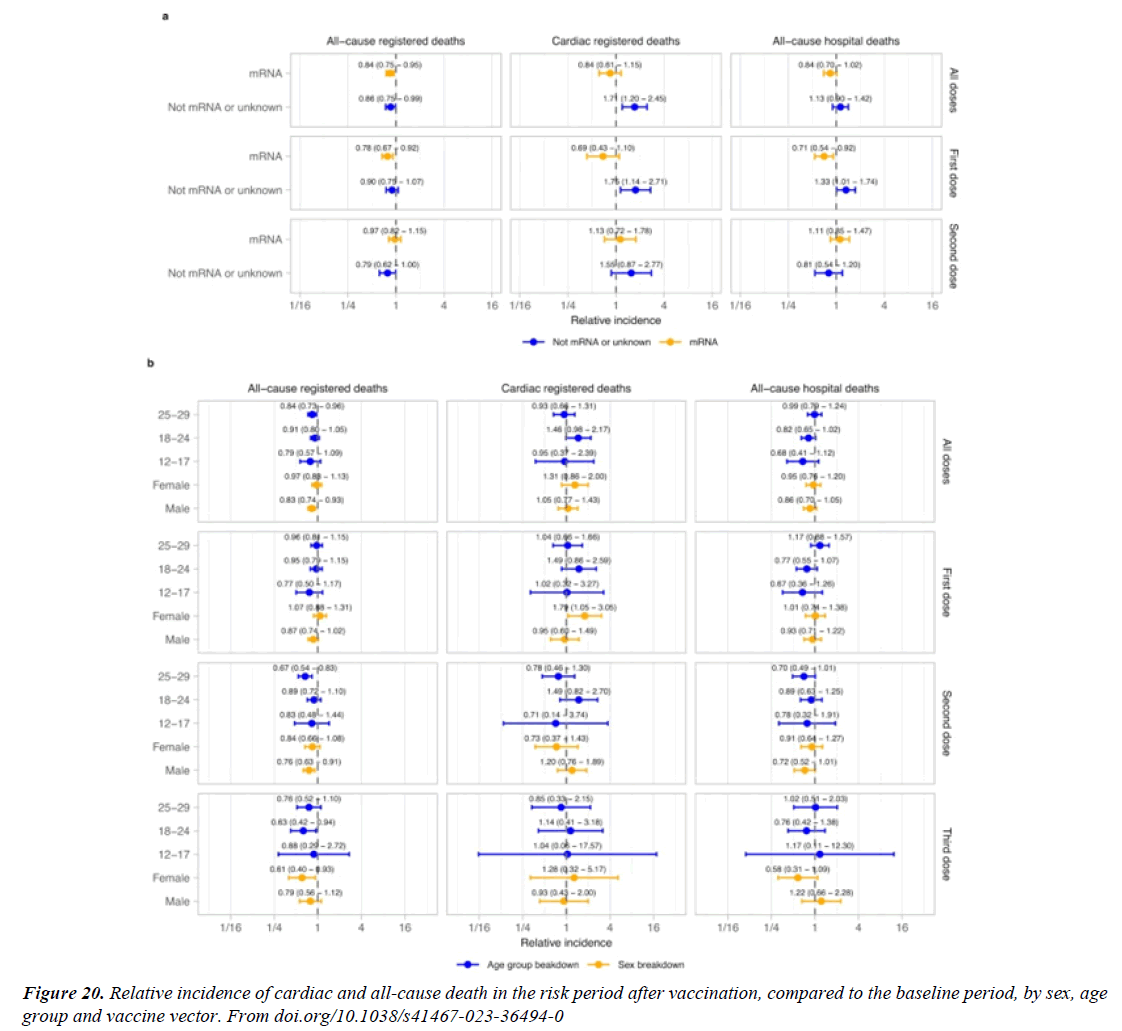
“A recent analysis of data from Florida (US) found increased risk of cardiac death in the first 4 weeks after mR.N.A. vaccination in people aged 18–39 years20 compared to subsequent weeks [12]. Cardiac-related deaths CRD following vaccination. In the 28 days following vaccination, a statistically significant increase in cardiac-related deaths was detected for the entire study population (RI = 1.07, 95% CI = 1.03 - 1.12). Stratifying by age group revealed RIs were significantly higher for age groups 25 - 39 (RI = 2.16, 95% CI = 1.35 - 3.47) and 60 or older (RI = 1.05, 95% CI = 1.01 - 1.10). The remaining age groups failed to reach statistical significance [13].
Myocarditis or pericarditis incidence after mR.N.A. COVID-19 vaccination in the current study (0–35.9 per 100,000 for males and 0–10.9 for females across age groups and vaccine cohorts) was similar to estimates found in a study from eight U.S. A. health systems in the Vaccine Safety Datalink (10). Previous CDC estimates found the highest risk for postvaccination myocarditis among males aged 16–17 years (10.6 per 100,000) during a 7-day risk window after receipt of a second mR.N.A. COVID-19 vaccine dose . Estimates from the current study (22.0 per 100,000 males aged 12–17 years) are higher, likely because outcomes were captured using ICD- 10-CM codes alone rather than through passive reporting with subsequent verification through medical record review. Among males aged 12–17 years, the group with the highest incidence of cardiac complications after receipt of a second mR.N.A. COVID-19 vaccine dose, the risk was 1.8–5.6 times as high after S.A.R.S.-CoV-2 infection than after the vaccination [14].
In 1987, Muller and his colleagues published a retrospective study of the death certificates of 2203 patients in Massachusetts who died outside of the hospital in 1983 . They found a circadian variation in sudden cardiac death SCD with 2 peaks during 24 h – the primary peak occurred from 7am to 11am and a secondary peak occurred between 5pm to 6pm. These results corresponded with the findings of the MILIS Study Group in 1985 that described the onset of acute myocardial infarction as following a circadian rhythm with a peak from 6am to noon [15]. This study work compared myocarditis mortality rate in the S.A.R.S.-CoV-2 vaccinated with that in the general population in Japan. The study was based on the materials and the vital statistics disclosed by the Japanese government.
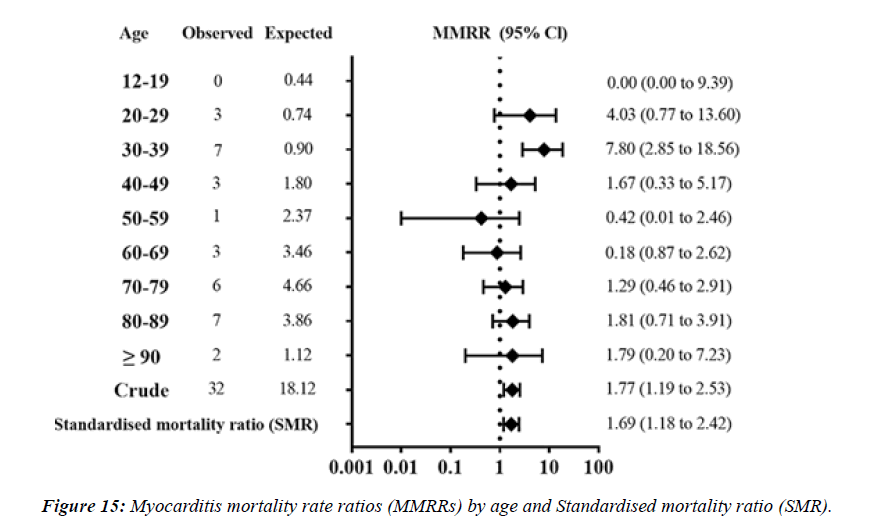
Number of myocarditis death MD which met the inclusion criteria were 32 cases. MMRR (95% confidence interval) was 4.03 (0.77 to 13.60) in 20s, 7.80 (2.85 to 18.56) in 30s, respectively. SMR of myocarditis was 1.69 (1.18 to 2.42) for overall vaccinated population, 1.35 (0.84 to 2.55) for those 60 years or older. Estimated adMMRRs and adSMR were about 4 times higher than the MMRRs and SMR. Pooled MOR for myocarditis were 148.49 (89.18 to 247.25).
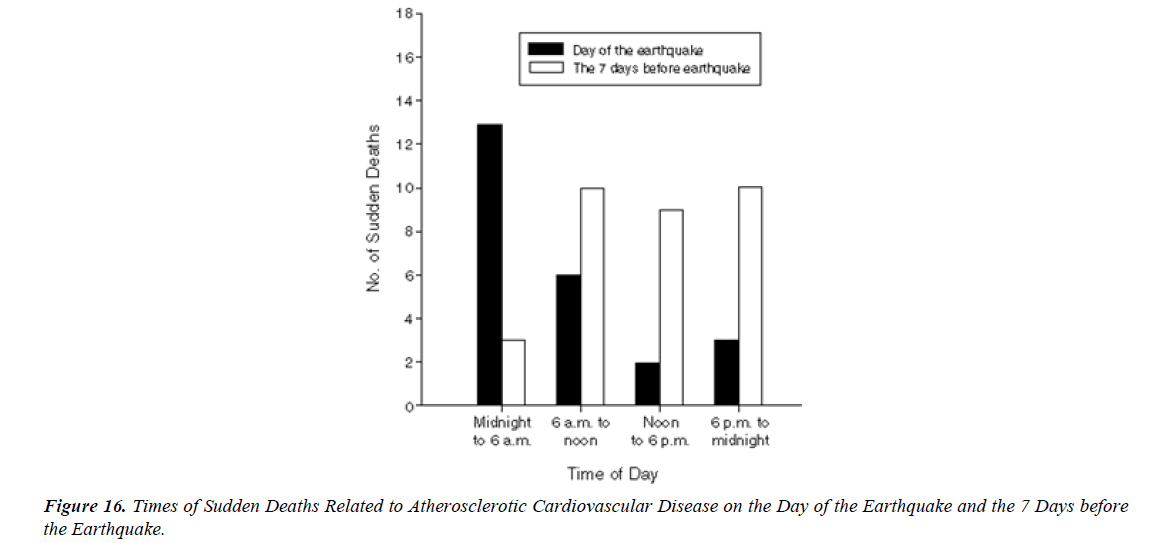
S.A.R.S.-CoV-2 vaccination was associated with higher risk of myocarditis death MD , not only in young adults but also in all age groups including the elderly. Considering healthy vaccinee effect, the risk may be 4 times or higher than the apparent risk of myocarditis death. Underreporting should also be considered. Based on this study, risk of myocarditis following S.A.R.S.-CoV-2 vaccination may be more serious than that reported previously [16].” There have been international reports, including from the US and Israel, of myocarditis and pericarditis following vaccination with COVID-19 mR.N.A. vaccines. Information to date indicates that these eventsoccur more commonly after the second dose, within the week following vaccination (typically within 4-5 days), mainly in adolescents/young adults and more often in males than females [17]. The mean age of the deceased was 62.6 years (age range: 32–91 years). Vaxzevria was vaccinated in 9, Comirnaty in 5 , Spikevax in 3, and Janssen in 1 person. The time interval between the last vaccination and death ranged from a few minutes (case 17) up to 14 days (case 9). Case 17 involved a person who collapsed in a vaccination center immediately after the vaccination; prompt resuscitation efforts were unsuccessful. A total of 12 deaths occurred at home, 4 deaths at a hospital, 1 death at a vaccination center, and 1 death at work [18]. Over half the deaths related to atherosclerosis (13 of 24) on the day of the earthquake occurred between midnight and 6 a.m., whereas only 3 of 32 (9 %) of the deaths during the 7 days before the earthquake occurred at that time of day (P = 0.002). The earthquake that struck the Los Angeles area at 4:31 a.m. on January 17, 1994, was 1 of the strongest earthquakes ever recorded in a major city in North America. In a huge analysis of more than 30,000 vaccinated patients who had experienced COVID breakthrough infections (pre- Omicron variant), scientists found that 6 months later, even the vaccinated incurred a higher risk of death and debilitating long COVID symptoms involving multiple organs ( lungs, heart, kidney, brain, others) when compared to controls without evidence of S.A.R.S.-CoV-2 infection. The postacute sequelae of S.A.R.S.-CoV-2 infection—also referred to as Long COVID—have been described, but whether breakthrough S.A.R.S.-CoV-2 infection (BTI) in vaccinated people results in post-acute sequelae is not clear. In this study, we used the US Dep. of Veterans Affairs national healthcare databases to build a cohort of 33,940 individuals with BTI and several controls of people without evidence of S.A.R.S.-CoV-2 infection, including contemporary (n = 4,983,491), historical (n = 5,785,273) and vaccinated (n = 2,566,369) controls. At 6 months after infection, we show that, beyond the first 30 days of illness, compared to contemporary controls, people with BTI exhibited a higher risk of death (hazard ratio (HR) = 1.75, 95% confidence interval (CI): 1.59, 1.93) and incident postacute sequelae (HR = 1.50, 95% CI: 1.46, 1.54), including cardiovascular CV, coagulation and hematologic, Gl, kidney, mental health, metabolic, musculoskeletal and neurologic disorders. The results were consistent in comparisons versus the historical and vaccinated controls. Compared to people with S.A.R.S.-CoV-2 infection who were not previously vaccinated (n = 113,474), people with BTI exhibited a lower risks of death (HR = 0.66, 95% CI: 0.58, 0.74) and incident post-acute sequelae (HR = 0.85, 95% CI: 0.82, 0.89). The findings suggest that vaccination before infection confers only partial protection in the post-acute phase of the disease [19]. Approximately 80% of all SCDs occur in the home and around 60% are witnessed” [20].
Circadian Variation in SCD
Circadian patterns have been the subject of considerable interest in CV , including both SCD as well as myocardial infarction MI. Cohen and coworkers analyzed 19 studies on the pattern of incidence of SCD. In their meta-analysis, including 19,390 patients, a morning excess in the incidence of SCD was found. Between 06:00 and 12:00, 30.1% of SCD events occurred; the incidence of SCD was 29% higher in the morning than during the rest of the day[21]. (relative risk 1.29; 95%”we find an increase in cardiac death in women after a first dose of non mR.N.A. vaccines. A positive S.A.R.S.- CoV-2 test is associated with increased cardiac and all-cause mortality among people vaccinated or unvaccinated at time of testing [22]. In the vaccination analysis, the vaccinated and control groups each included a mean of 884,828 persons. Vaccination was most strongly associated with an elevated risk of myocarditis (risk ratio, 3.24; 95% confidence interval [CI], 1.55 to 12.44; risk difference, 2.7 events per 100,000 persons; 95% CI, 1.0 to 4.6). S.A.R.S.-CoV-2 infection was associated with a substantially increased risk of myocarditis (risk ratio, 18.28; 95% CI, 3.95 to 25.12; risk difference, 11.0 events per 100,000 persons; 95% CI, 5.6 to 15.8) and of additional serious adverse AE events, including pericarditis, arrhythmia, deep-vein thrombosis, pulmonary embolism EP , myocardial infarction, intracranial hemorrhage, and thrombocytopenia. The vaccine was associated with an excess risk of myocarditis (1 to 5 events per 100,000 persons). The risk of this potentially serious adverse event and of many other serious adverse events was substantially increased after S.A.R.S.-CoV-2 infection [23]. There were 19,740,741 doses of mR.N.A. vaccines administered and 297 reports of myocarditis/ pericarditis meeting our inclusion criteria. 69.7% occurred following the second dose of COVID-19 mR.N.A. vaccine and 76.8% occurred in males. The median age of individuals with a reported event was 24 years. The highest reporting rate of myocarditis/pericarditis was observed in males aged 18-24 years following mR.N.A.-1273 as the second dose; the rate in this age group was 5.1 (95% CI 1.9-15.5) times higher than the rate following BNT162b2 as the second dose. Overall reporting rates were higher when the inter-dose interval was shorter ( ≤30 days) for both vaccine products. Among individuals who received mR.N.A.-1273 for the second dose, rates were higher for those who had a heterologous as opposed to homologous vaccine schedule [24].
We analysed 196 death cases reported after inoculation of Pfizer-BioNTech COVID-19 vaccine (COMIRNATY) by June 9 in Japan. Japanese Ministry of Health JMH , Labour, and Welfare virtually denied the causality of all cases without conducting appropriate epidemiological analysis.
Mortality odds ratio was calculated as the indicator of disproportionality in cause of death. We compared odds of cause of death after inoculation and death in Japanese vital statistics in 2019 as control non-vaccinated population. MOR was obtained by using the numbers of death from non- CV system as the reference causes for 2 age groups: vaccinated medical workers (20 to 74 year of age) and elderly (≧ 65years) separately.
Of 31 deaths among vaccinated medical workers (both sexes), 26 (84%) died from CV diseases, such as stroke, myocardial infarction, venous thrombosis and pulmonary embolism (VT/PE) and heart failure, while 22% died in the general population. MOR is 19.4 (p<0.0001). MOR of hemorrhagic stroke (40.7) and VT/PE (114.0) were extremely high. The reported vaccinated elderly death cases, 69% died from CV causes, while 26% died in the general population. MOR is 5.9 (p<0.0001). MOR of hemorrhagic stroke (12.8) and VT/ PE (24.9) were also very high.These suggest that COVID-19 vaccination is closely associated with the risk of death from CV causes,especially hemorrhagic stroke and VT/PE [25].
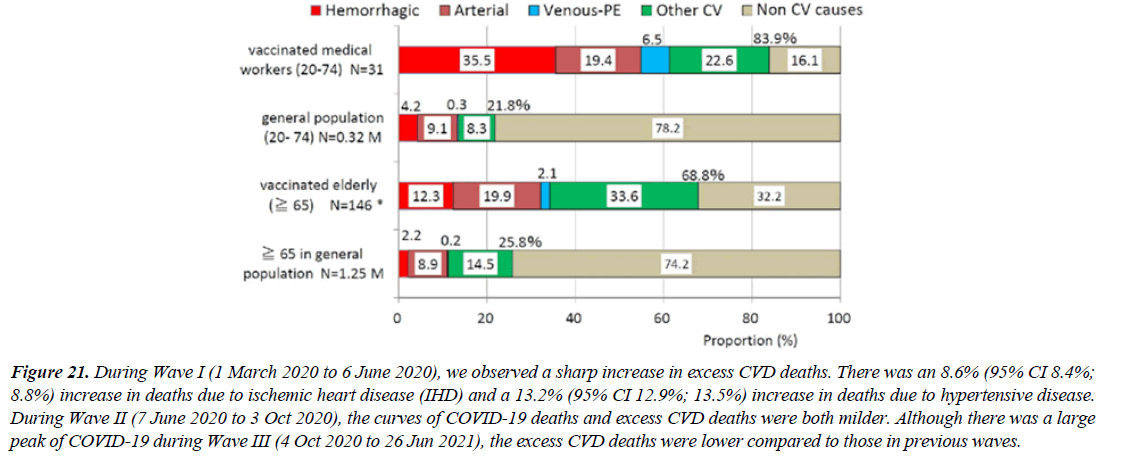
But An adverse event occurring after vaccination doesn't prove vaccination caused it. During Wave I (1 March 2020 to 6 June 2020), we observed a sharp increase in excess CVD deaths. There was an 8.6% (95% CI 8.4%; 8.8%) increase in deaths due to ischemic heart disease (IHD) and a 13.2% (95% CI 12.9%; 13.5%) increase in deaths due to hypertensive disease. During Wave II (7 June 2020 to 3 Oct 2020), the curves of COVID-19 deaths and excess CVD deaths were both milder. Although there was a large peak of COVID-19 during Wave III (4 Oct 2020 to 26 Jun 2021), the excess CVD deaths were lower compared to those in previous waves [26]. A literature review of COVID-19-vaccine-related deaths has been carried out according to PRISMA standards to understand if there is a causal relationship between vaccination and death and to highlight the real extent of such events.
There have been 55 cases of death after COVID-19 vaccination reported and a causal relationship has been excluded in 17 cases. In the remaining cases, the causal link between the vaccine and the death was not specified (8) or considered possible (15), probable (1), or very probable/ demonstrated (14). The causes of deaths among these cases were: vaccine-induced immune thrombotic thrombocytopenia (32), myocarditis (3), ADEM (1), myocardial infarction (1), and rhabdomyolysis [27]. In this study work in a nationwide mass vaccination setting, the BNT162b2 vaccine was not associated with an elevated risk of most of the adverse events examined. The vaccine was associated with an excess risk of myocarditis (1 to 5 events per 100,000 persons). The risk of this potentially serious adverse event and of many other serious adverse events was substantially increased after S.A.R.S.- CoV-2 infection 62 studies, including 218 cases, participated in the current systematic review [28]. The median age was 29.2 years; 92.2% were male and 7.8% were female. 72.4% of patients received the Pfizer-BioNTech (BNT162b2) vaccine, 23.8% of patients received the Moderna COVID-19 Vaccine (mR.N.A.-1273), and the rest of the 3.5% received other types of COVID-19 vaccine. Most myocarditis cases (82.1%) occurred after the second vaccine dose, after a median time interval of 3.5 days. The most frequently reported symptoms were chest pain, myalgia/body aches and fever. The Troponin levels were consistently elevated in 98.6% of patients. The admission ECG was abnormal in 88.5% of cases, and the left LVEF was lower than 50% in 21.5% of cases. Most patients (92.6%) resolved symptoms and recovered, and only 3 patients died [29]. The Israeli Ministry of Health reported 62 cases of myocarditis in patients vaccinated for COVID-19 out of 5 million vaccinated individuals. Great number cases occurred after the second dose of mR.N.A. vaccines, with only 6 cases diagnosed after the first dose. The prevalence was higher in men under 30 years of age, increasing from 1/100 000 for the general population, to 1/20 000 for the 16-30 years old group. 2 of the 62 patients died. The U.S. Department of Defense reported 14 military personnel diagnosed with myocarditis following COVID vaccination, 13 of them after their second dose of COVID-19 mR.N.A. vaccines [30].
Experimental project hypotesys
Because the toxicity of S.A.R.S.-cov-2 spike protein and its derivates is knowed it is of interest to verify. The incidence and prevalence of Sudden cardiac deaths in a determinate geographic population.before covid-19 pandemia During pandemia and after covid disease with out vaccination During pandemia after covid -19 disease and with vaccination After pandemia whit only vaccination ( no disease) This data make possible to verify the contribution of every single movens.
Discussion
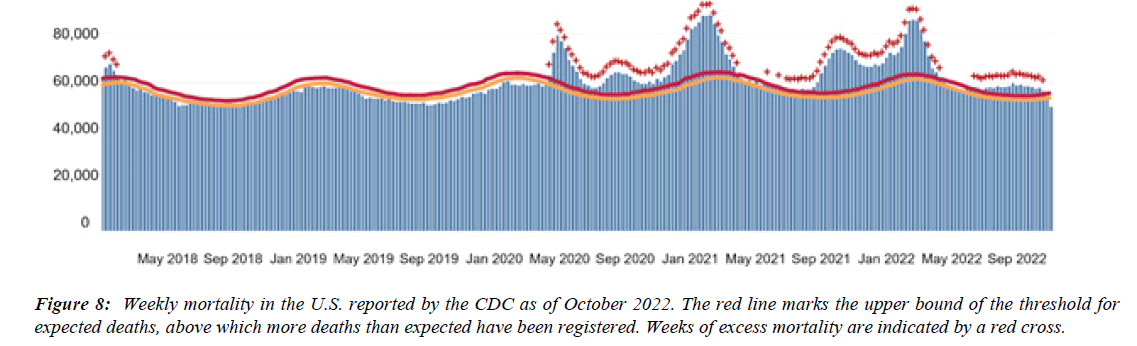
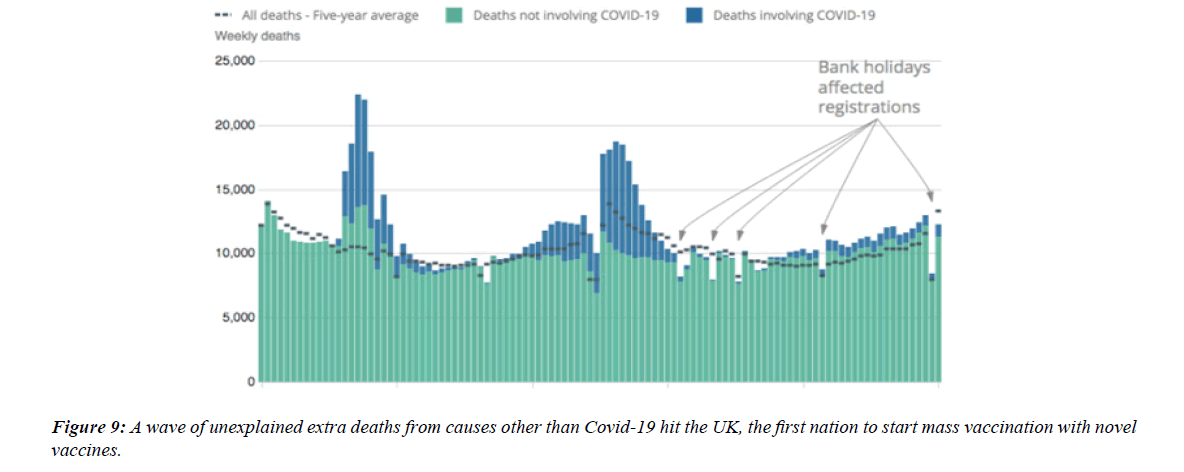
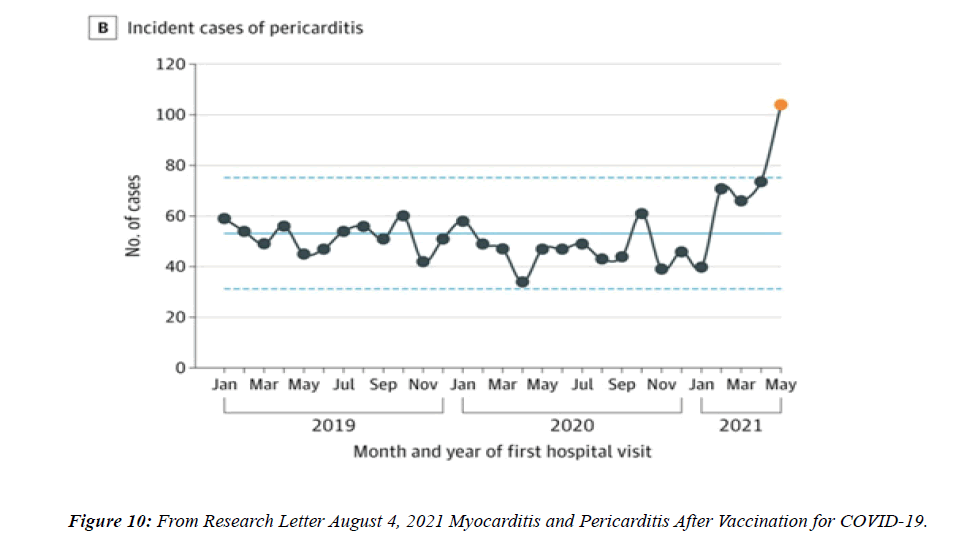
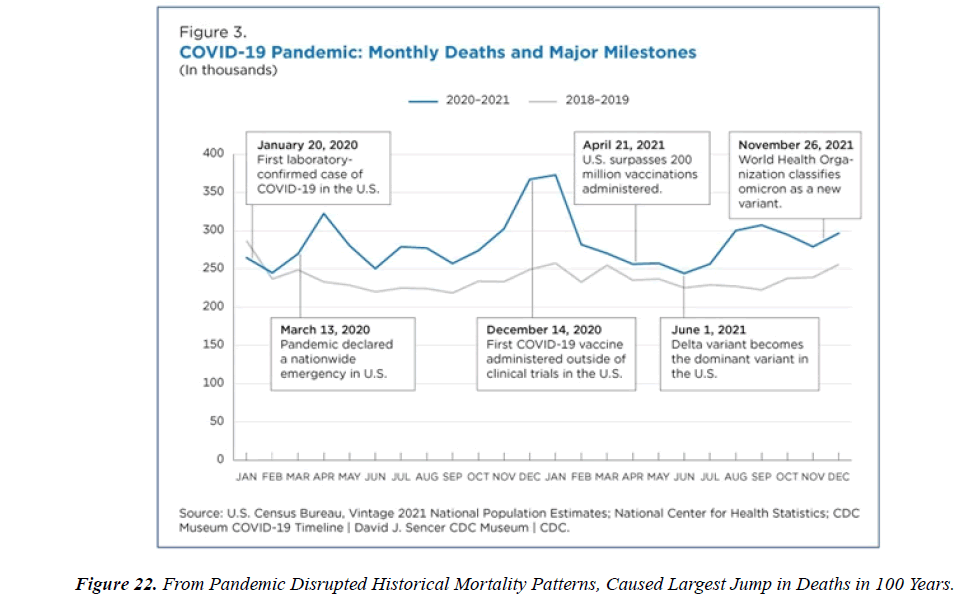
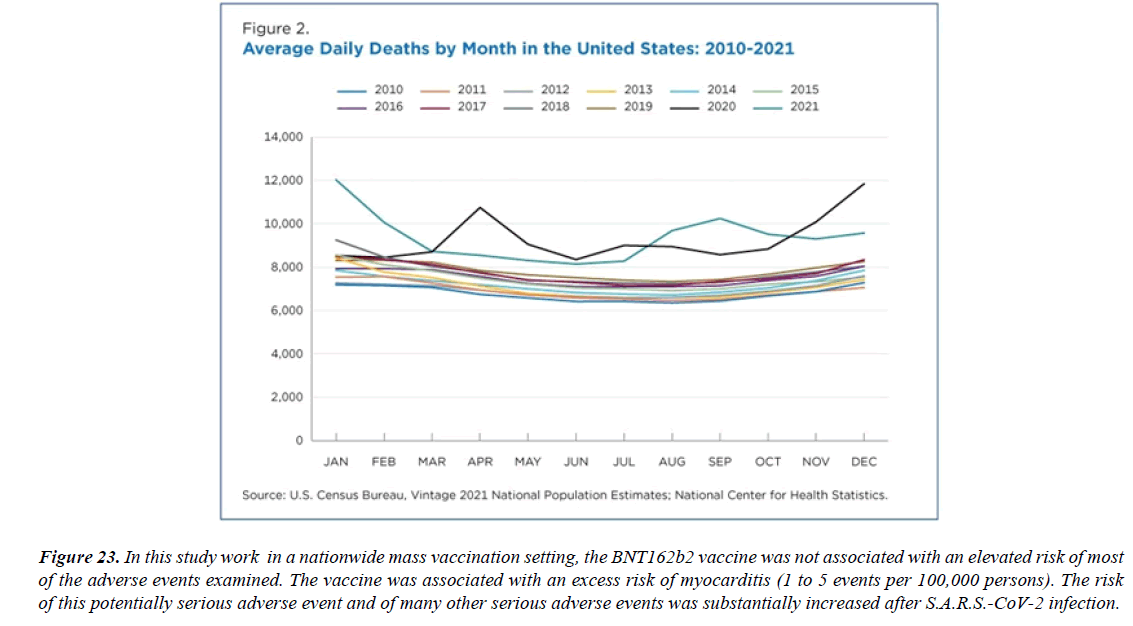
The profile of CV complication of covid-19 disease is clearly reported by literature. Related reference reported it is possible to verify this temporal presentation of effects: The same the infected patient (with severe disease) show increased level of cardio vascular complication vs the non-infected. (ESC 2023) This data was higher vs prepandemic periods. (Xie, Y. Et al ) In figure 20 the montly death 2020-21 was always superior then in 2018-19 years even if after starting the mass vaccinations. The same the montly death in years from 2010 to 2019 was lower than 2020-21. Great part of sudden deaths ar of cardiovascular origin. (HUNTER P.) Extradeaths are reported from jan 2021. A part from covid-19 disease deaths are registered extradeaths. (feb- july 2022 UK- figure 3) Pericarditis increased from May 2021 (USA) ,Cardiac arrest call reported peaks on 2021 march and july interest the circadian profile of CSD and the days of presentation after vaccination / when happened and registerd ). Great part of deaths after covid -19 vaccination happened in less then 3 days. Of interest also to observe the pathological mechanism of action of S.A.R.S. cov-2 spike protein trought the link with ACE2 receptor in pulmonary and other tissue .The same to observe the RARE ADR of some m RNA covid-19 vaccine like pericarditys and miocarditys in young males. (See literaure published). Of interest to observe the role played by ACE INIBITHORS in miocarditys therapy. The incidence of CV DEATHS or unexpected deaths before and after the covid-19 vaccination campaign is an interesting phenomenon Trends from 2012 to 2017 are reported in references (not great increased)
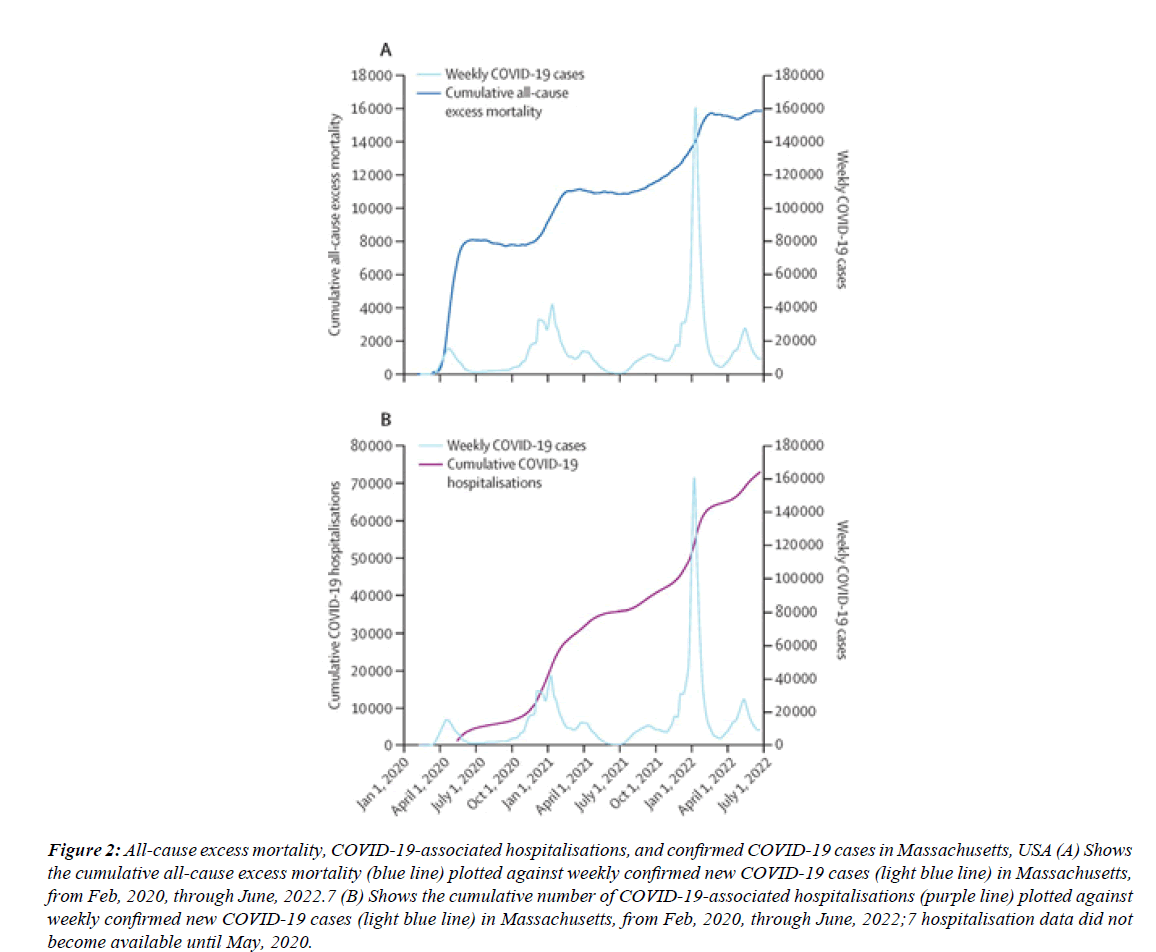
Figure 2: All-cause excess mortality, COVID-19-associated hospitalisations, and confirmed COVID-19 cases in Massachusetts, USA (A) Shows the cumulative all-cause excess mortality (blue line) plotted against weekly confirmed new COVID-19 cases (light blue line) in Massachusetts, from Feb, 2020, through June, 2022.7 (B) Shows the cumulative number of COVID-19-associated hospitalisations (purple line) plotted against weekly confirmed new COVID-19 cases (light blue line) in Massachusetts, from Feb, 2020, through June, 2022;7 hospitalisation data did not become available until May, 2020.
Figure 5:A Frequencies of SCN5A variants in our cohort with sudden unexplained death (SUD) cases within 7 days of COVID-19 vaccination, in Thai SUD cases during 8–30 days after COVID-19 vaccination during the same period, in a Thai cohort of SUD before the COVID-19 pandemic, and in our in-house exome database enrolling patients and their parents with various rare diseases. B: The odds ratios OR and 95% confidence intervals for SCN5A variants and SUD in study groups, compared with the in-house exome controls as a reference. The asterisk indicates that the P-value reaches statistical significance of less than .05. From DOI:https://doi.org/10.1016/j.hrthm.2022.07.019..
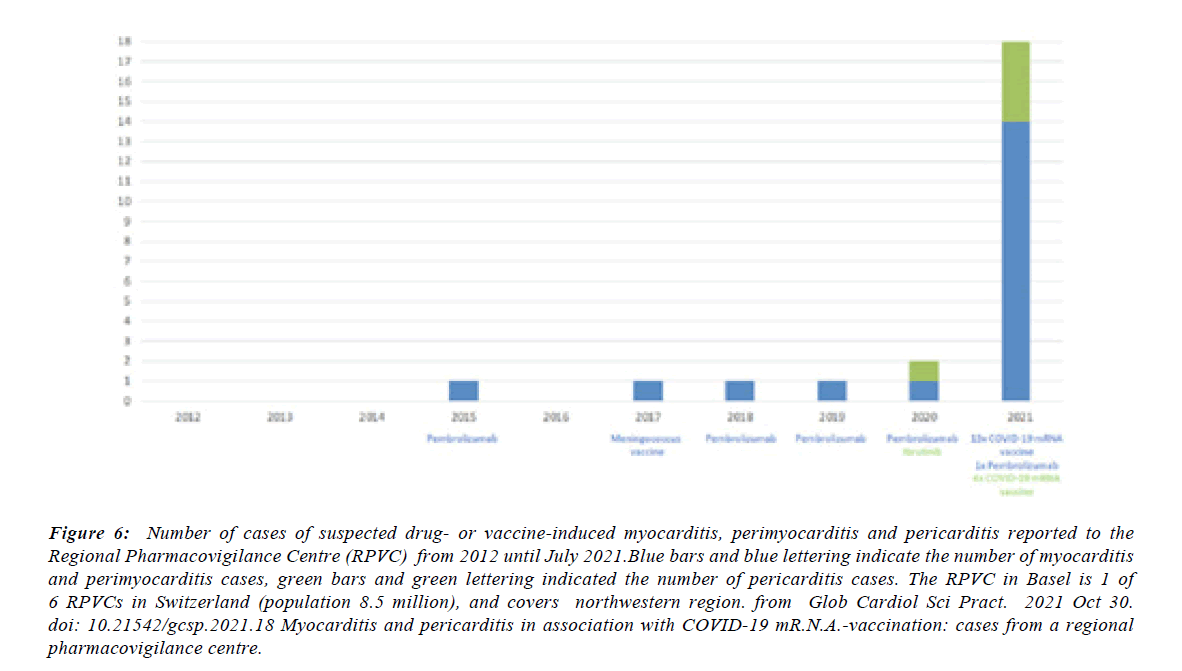
Figure 6:Number of cases of suspected drug- or vaccine-induced myocarditis, perimyocarditis and pericarditis reported to the Regional Pharmacovigilance Centre (RPVC) from 2012 until July 2021.Blue bars and blue lettering indicate the number of myocarditis and perimyocarditis cases, green bars and green lettering indicated the number of pericarditis cases. The RPVC in Basel is 1 of 6 RPVCs in Switzerland (population 8.5 million), and covers northwestern region. from Glob Cardiol Sci Pract. 2021 Oct 30. doi: 10.21542/gcsp.2021.18 Myocarditis and pericarditis in association with COVID-19 mR.N.A.-vaccination: cases from a regional pharmacovigilance centre.
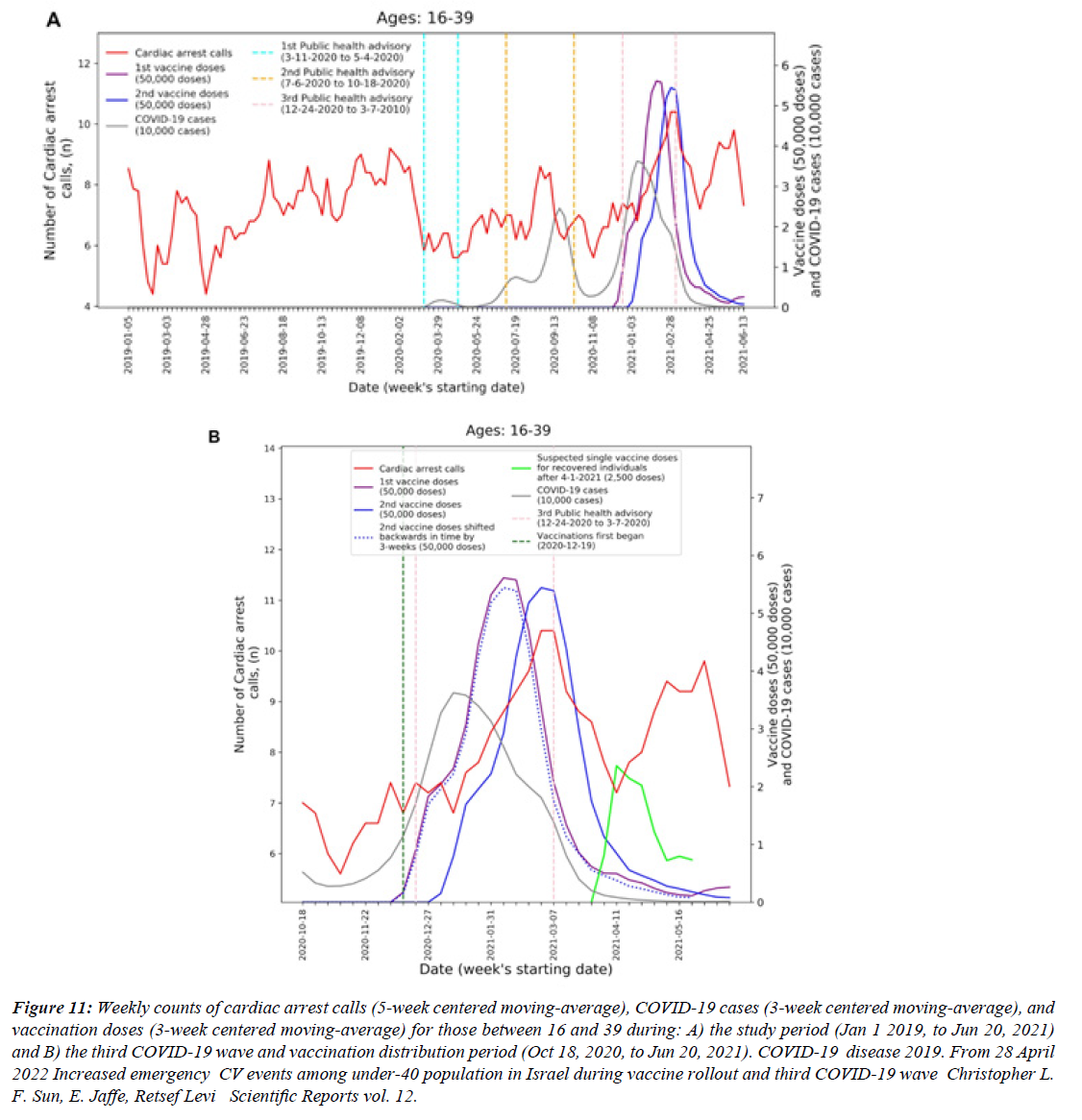
Figure 12:Weekly counts of cardiac arrest calls (5-week centered moving-average), COVID-19 cases (3-week centered moving-average), and vaccination doses (3-week centered moving-average) for those between 16 and 39 during: A) the study period (Jan 1 2019, to Jun 20, 2021) and B) the third COVID-19 wave and vaccination distribution period (Oct 18, 2020, to Jun 20, 2021). COVID-19 disease 2019. From 28 April 2022 Increased emergency CV events among under-40 population in Israel during vaccine rollout and third COVID-19 wave Christopher L. F. Sun, E. Jaffe, Retsef Levi Scientific Reports vol. 12.
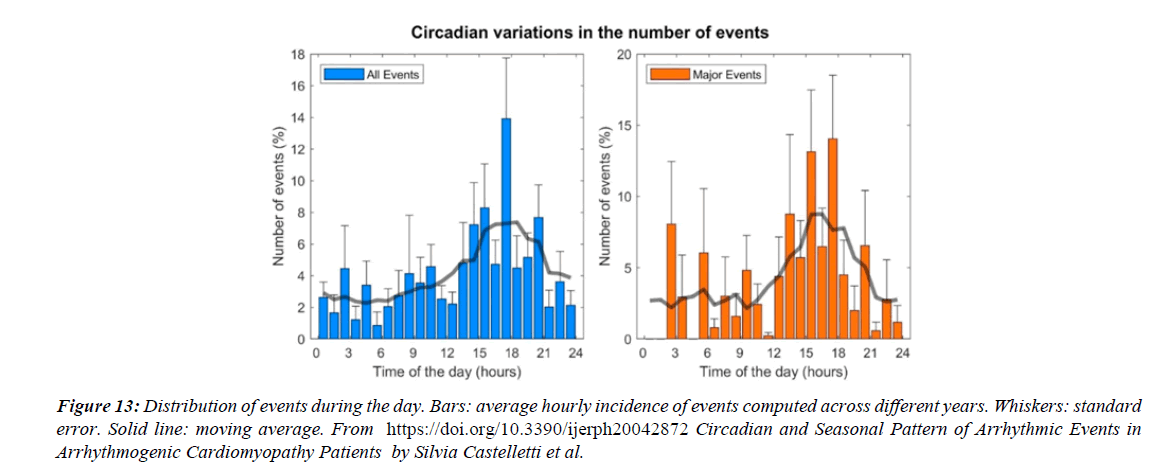
Figure 13:Distribution of events during the day. Bars: average hourly incidence of events computed across different years. Whiskers: standard error. Solid line: moving average. From https://doi.org/10.3390/ijerph20042872 Circadian and Seasonal Pattern of Arrhythmic Events in Arrhythmogenic Cardiomyopathy Patients by Silvia Castelletti et al.
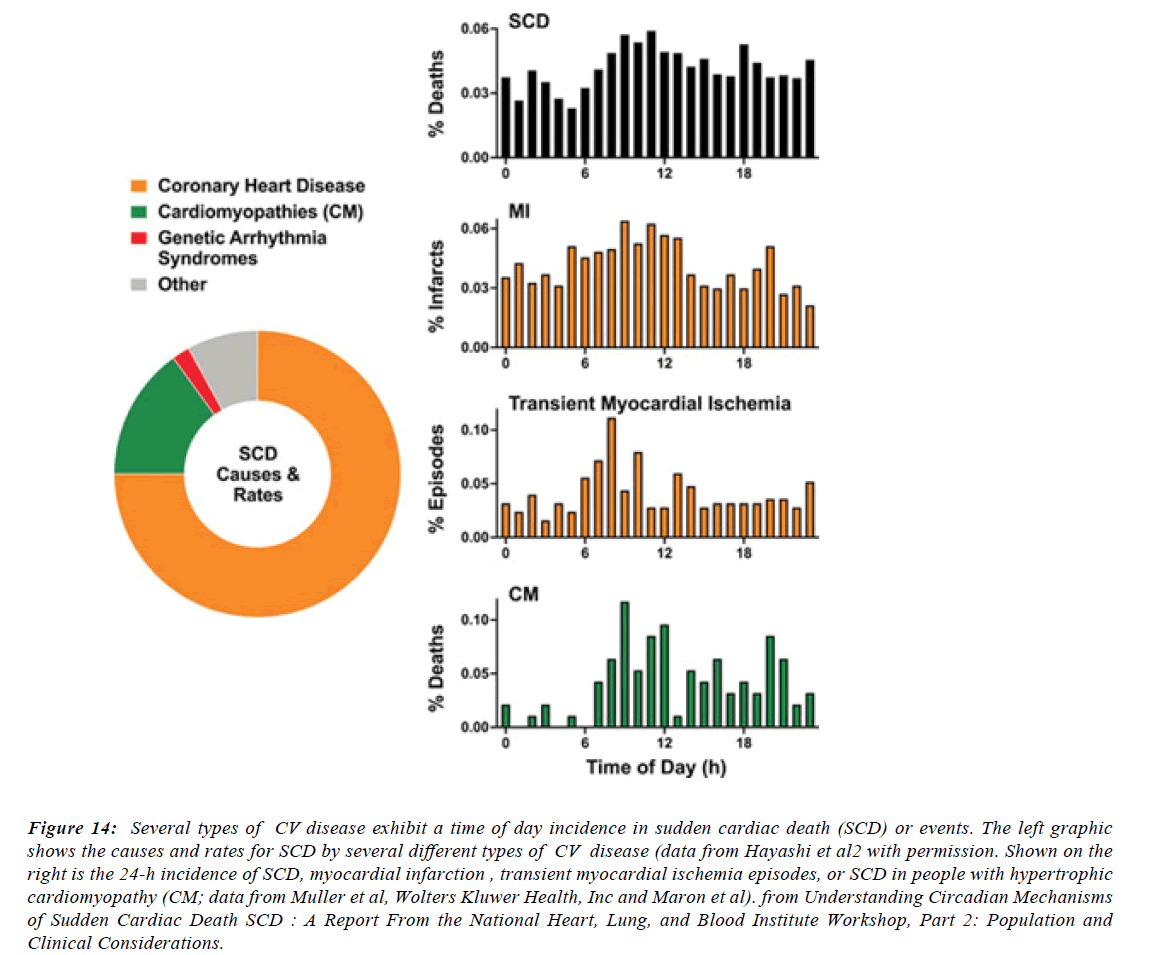
Figure 14:Several types of CV disease exhibit a time of day incidence in sudden cardiac death (SCD) or events. The left graphic shows the causes and rates for SCD by several different types of CV disease (data from Hayashi et al2 with permission. Shown on the right is the 24-h incidence of SCD, myocardial infarction , transient myocardial ischemia episodes, or SCD in people with hypertrophic cardiomyopathy (CM; data from Muller et al, Wolters Kluwer Health, Inc and Maron et al). from Understanding Circadian Mechanisms of Sudden Cardiac Death SCD : A Report From the National Heart, Lung, and Blood Institute Workshop, Part 2: Population and Clinical Considerations.
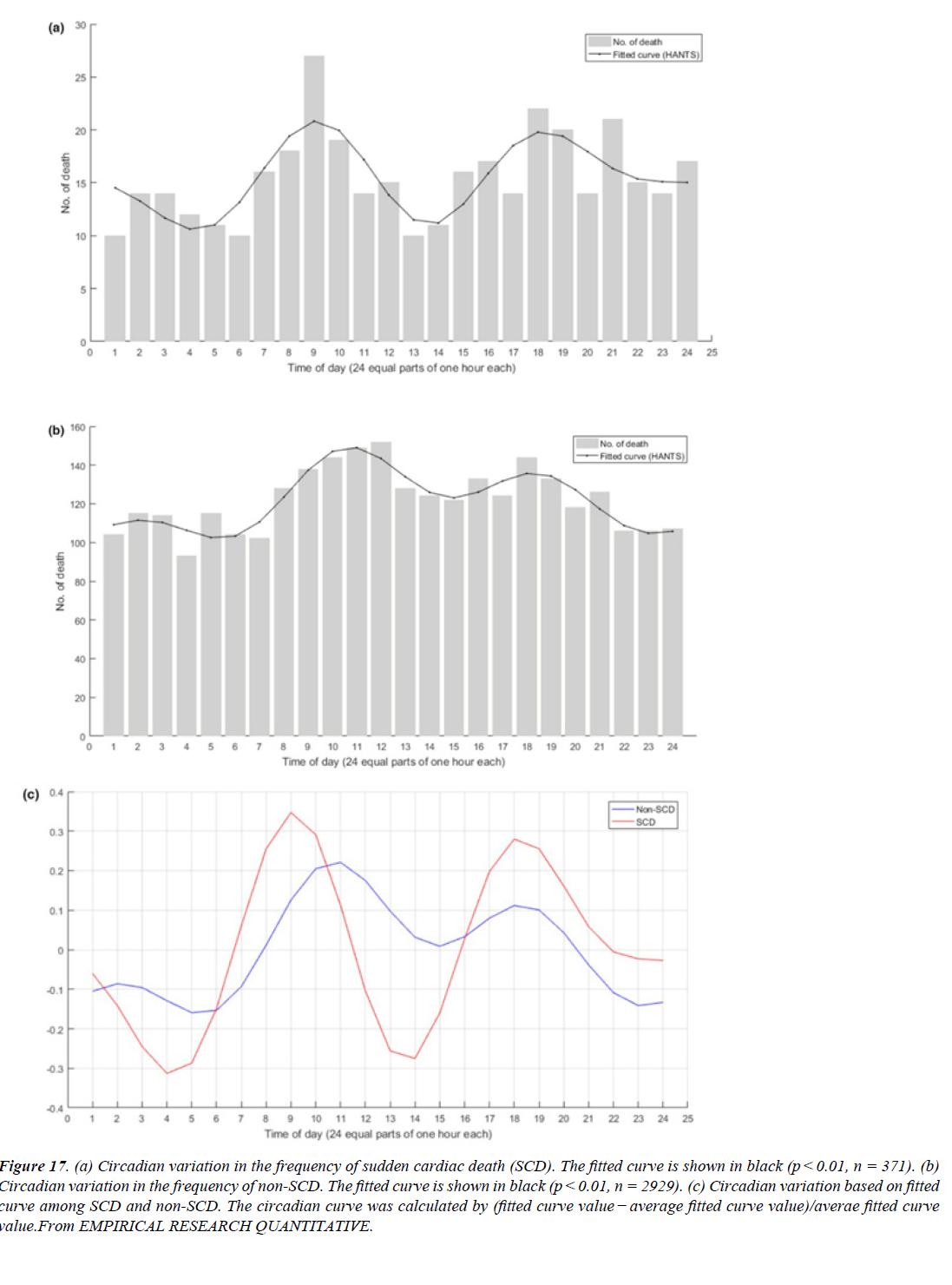
Figure 17:(a) Circadian variation in the frequency of sudden cardiac death (SCD). The fitted curve is shown in black (p < 0.01, n = 371). (b) Circadian variation in the frequency of non-SCD. The fitted curve is shown in black (p < 0.01, n = 2929). (c) Circadian variation based on fitted curve among SCD and non-SCD. The circadian curve was calculated by (fitted curve value - average fitted curve value)/averae fitted curve value.From EMPIRICAL RESEARCH QUANTITATIVE.
Figure 21:During Wave I (1 March 2020 to 6 June 2020), we observed a sharp increase in excess CVD deaths. There was an 8.6% (95% CI 8.4%; 8.8%) increase in deaths due to ischemic heart disease (IHD) and a 13.2% (95% CI 12.9%; 13.5%) increase in deaths due to hypertensive disease. During Wave II (7 June 2020 to 3 Oct 2020), the curves of COVID-19 deaths and excess CVD deaths were both milder. Although there was a large peak of COVID-19 during Wave III (4 Oct 2020 to 26 Jun 2021), the excess CVD deaths were lower compared to those in previous waves.
Figure 23:In this study work in a nationwide mass vaccination setting, the BNT162b2 vaccine was not associated with an elevated risk of most of the adverse events examined. The vaccine was associated with an excess risk of myocarditis (1 to 5 events per 100,000 persons). The risk of this potentially serious adverse event and of many other serious adverse events was substantially increased after S.A.R.S.-CoV-2 infection.
Related the data published it is possible to consider that the similar population pre and post pandemia studied have an equal level of basic cardio vascular pathology , so the additional mortality can not to be considered due only by this phenomena. To be considered also that various references report that after vaccination some event are registered in healthy patients
Conclusion
Even Considering that the profilaxys measure like vaccination are useful in various virus disease observing scientific database some question are of interest. For the meaning of this work it is necessary read the data and figure related CSD or deaths considering prepandemic years, pandemic season and also the vaccine campain timing ( first doses and second or other doses). Why montly mortality rate was higher in 2021 then in 2013 in USA ? What is the cumulative effect played on Sudden cardiac extra deaths and unexpected by Covid disease or Long covid added to vaccination ? And due only by vaccination?It is possible to choose 2013 data as baseline. This information are usefull to better understand this global phenomena .Why great amount of unexpected extradeath was observed at home ? The figure reported in this work added to the time presentation of the effect can give Information about this phenomena .The fact that was registered exatradeaths in last periods a part the classic parameter ( 2012-2017) can be Due by the effect of the pandemia ( or to long covid) but it must to be more deeply investigated also the effect played by the spike protein derivates (wide used )to fight this virus.(are there relationship?). See ref 30, military often is healthy. Some producers of covid-19 vaccine introduced in covid vaccine -technical sheet information about risk of rare myocarditys in subpopulations. Relevant the study related the SUD in Brugada syndrome associated a covd-19 vaccination. (genetic factors involved). The vaccination after the covid disease can be considered like and additional dose of Spike protein. (Ref n .5 for the cumulative effect). The incidence of myocarditys after some covid-19 vaccination was higer in some subclasses (young then adults). Event like pericarditis and myocarditys increase after the second dose rates were higher when the inter-dose interval was shorter (i.e., ≤30 days). Interest to observe taht mortality increase in statistical way every year due by the difference between new born and new elderly (so increase elderly and relted deaths) The average data of increase is greater then of the 2015-19. The difference between 2022 and 2021 can be used a measure of the vaccine influence. In the 2022it was observed an +12% vs 2021.The fact that in 2020 was reported more deaths can be related to various factors ( Pandemia, kind of preventive measures adopted and kind of therapy choosen). Literature published provided evidence of this. If mortality in 2020 was only due to sars-cov-2 virus and not other co-factor (also by some kind of preventive measure, drugs used or not, protocol adopted in emergency) in the 2021-2022 it hd to be observed to an reduction: instead the cause of death for all causes was increased. (See literature published and reported and also statistical data published by pubblic org.)
Conflict of interest
No
References
- Cadegiani FA. Catecholamines are the key trigger of COVID-19 mRNA vaccine-induced myocarditis: A compelling hypothesis supported by epidemiological, anatomopathological, molecular, and physiological findings. Cureus. 2022;14(8).
- Choi S, Lee S, Seo JW, et al. Myocarditis-induced sudden death after BNT162b2 mRNA COVID-19 vaccination in Korea: Case report focusing on histopathological findings. J Korean Med Sci. 2021;36(40).
- Vaartjes I, Hendrix A, Hertogh EM, et al. Sudden death in persons younger than 40 years of age: Incidence and causes. Eur J Prev Cardiol. 2009;16(5):592-6.
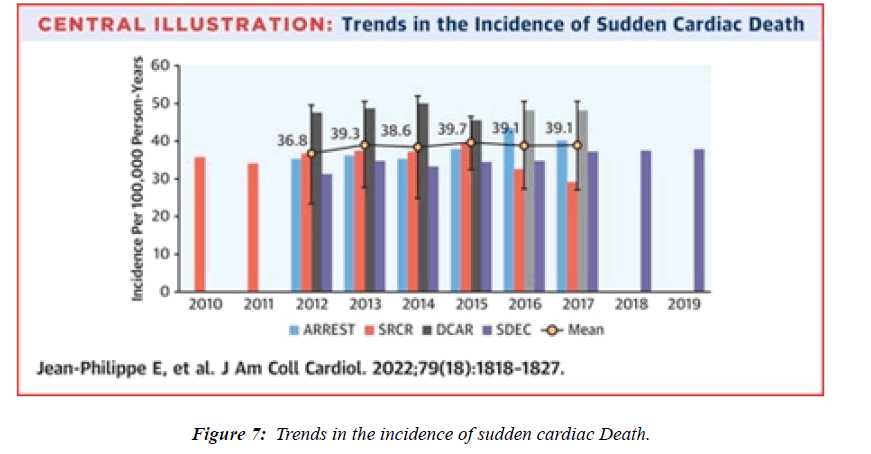
- Empana JP, Lerner I, Valentin E, et al. Incidence of sudden cardiac death in the European Union. J Ame Coll of Cardi. 2022;79:1818-27
- Akhtar Z,Trent M, Moa A. The impact of COVID-19 and COVID vaccination on cardiovascular outcomes. Eur Heart J Suppl. 2023; A42-9.
- Bozkurt B, Kamat I, Hotez PJ. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021;(6):471-84.
- Hong W, Tang W. Myocarditis Medication. Drugs & Dis. 2021.
- Choi S, Lee SH, Seo JW. Myocarditis-induced sudden death after BNT162B2 mRNA COVID-19 vaccination in Korea: Case report focusing on histopathological findings. J Korean Med Sci. 2021;1477789.
- Mansanguan S, Charunwatthana P, Piyaphanee W, Cardiovascular manifestation of the BNT162b2 mRNA COVID-19 vaccine in adolescents. Trop Med Infect Dis. 2022.
- Schwab C, Domke LM, Hartmann L. Autopsy-based histopathological characterization of myocarditis after anti-S.A.R.S.-CoV-2-vaccination. Clin Res Cardiol. 2023;(3):431-40.
- Peñaloza-Martínez E, Moreno G, Aroca-Crevillén A. Circadian rhythms in thrombosis and atherothrombotic events. Front Biosci. 2022;(2):51.
- Nafilyan V, Bermingham CR, Ward IL. Risk of death following COVID-19 vaccination or positive S.A.R.S.-CoV-2 test in young people in England. Nat Commun. 2023;14:1541.
- Exploring the relationship between all-cause and cardiac-related mortality following COVID-19. 2022;1-8.
- Centers for Disease Control (US). Morbidity and mortality weekly report: MMWR. US Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control; 1979.
- Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination-PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-23.
- Ramireddy A, Chugh SS. Do peak times exist for sudden cardiac arrest? Trends Cardiovasc Med. 2021; 172-76.
- Watanabe S, Hama R. Preprint SARS-CoV-2 vaccine and increased myocarditis mortality risk: A population based comparative study in Japan. medRxiv. 2022.
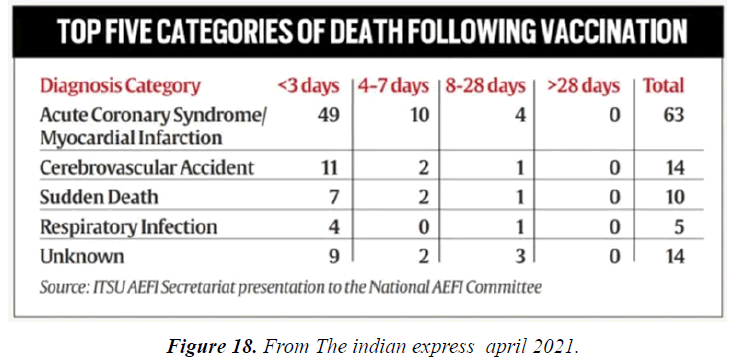
- Adverse Events Following Immunization (AEFIs) for COVID-19 in Ontario: December 13, 2020 to
- Schneider J, Sottmann L, Greinacher A. Postmortem investigation of fatalities following vaccination with COVID-19 vaccines.OMS. 2021; 2335-45.
- Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022; 1461-67.
- Adabag AS, Luepker RV, Roger VL, et al. Sudden cardiac death: Epidemiology and risk factors. Nat Rev Cardiol. 2010;(4): 216-25.
- Mahmoud KD, de Smet BJGL, Zijlstra F, et al. Sudden cardiac death: Epidemiology circadian variation, and triggers. Curr Probl Cardiol. 2011;(2):56-80.
- Nafilyan V, Bermingham CR, Ward IL. et al. Risk of death following COVID-19 vaccination or positive S.A.R.S.-CoV-2 test in young people in England. Nat Commun. 2023.
- Barda N, Ben-Shlomo Y, Waxman J. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide Setting. N Engl J Med. 2021.
- Buchan S, Yon Seo C, Johnson C. Epidemiology of myocarditis and pericarditis following mRNA vaccination by vaccine product, schedule, and interdose interval among adolescents and adults in ontario, Canada. JAMA Netw Open. 2022;(6):e2218505.
- COVID-19 vaccine: Strong association with cardiovascular death,especially hemorrhagic stroke and venous thrombosis. Editorial.
- Han L, Zhao S, Li S, et al. Excess cardiovascular mortality across multiple COVID-19 waves in the United States from March 2020 to March 2022. Nat Cardiovasc Res. 2023;2(3):322-33.
- Maiese A, Baronti A, Manetti AC, et al. Death after the administration of COVID-19 vaccines approved by EMA: Has a causal relationship been demonstrated? Vaccines. 2022;10(2):308.
- Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021.
- Ahmed SK, Mohamed MG, Essa RA, et al. Global reports of myocarditis following COVID-19 vaccination: A systematic review and meta-analysis. Diabetes Metab Syndr. 2022;102513.
Albert E, Aurigemma G, Saucedo J, et al. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021;16(8):2142-5.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
March 26, 2023.1-36.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.