Mini Review - Journal of Neurology and Neurorehabilitation Research (2023) Volume 8, Issue 3
Study of the Relationship between Vitamin D Status in Acute Ischemic Stroke and Initial Severity and Short-Term Outcome in a Tertiary Level Hospital, Bangladesh
Aminur Rahman1, Muhammad Jamil Ahmed2, Abul Hasnat Russel2, Mohammed Nazmul Huq3, Md Nurul Amin Miah4, Zahed Ali11Department of Neurology, Sir Salimullah Medical College, Dhaka, Bangladesh
2Department of Neurology, Sir Salimullah Medical College Mitford Hospital, Dhaka, Bangladesh
3Department of Statistics, Jahangirnagar University, Savar, Dhaka, Bangladesh
4Department of Medicine, Sheikh Hasina Medical College Tangail, Bangladesh
- *Corresponding Author:
- Aminur Rahman
Department of Neurology
Sir Salimullah Medical College
Dhaka, Bangladesh
E-mail: draminur@yahoo.com
Received: 04-Jun-2023, Manuscript No. AAJNNR-23-85832; Editor assigned: 06-Jun-2023, Pre QC No. AAJNNR-23-85832(PQ); Reviewed: 20-Jun-2023, QC No. AAJNNR-23-85832; Revised: 22-Jun-2023, Manuscript No. AAJNNR-23-85832(R); Published: 29-Jun-2023, DOI: 10.35841/aajnnr-8.3.141
Citation: Rahman A, Ahmed JM, Russel HA, Huq NM, Miah AN, Ali Z. Study of the relationship between vitamin d status in acute ischemic stroke and initial severity and short-term outcome in a tertiary level hospital, Bangladesh. J Neurol Neurorehab Res. 2023;8(3):141
Abstract
Background: As per recent research, vitamin D, a neuroprotective prohormone, may serve as a defense against neurovascular injury. Low vitamin D levels had a modest risk of stroke and fatal stroke. Objective: This research aimed to see the relationship between vitamin D status relates to initial severity and short-term outcome in patients with acute ischemic stroke. Methods: The study included 51 patients with acute ischemic stroke and 51 matched healthy control participants. According to their levels of vitamin D, the subjects were split into three groups: deficient, insufficient, and sufficient. All patients underwent the modified Rankin Scale (mRS) at discharge and after three months, as well as the National Institutes of Health Stroke Scale (NIHSS) during admission and after 72 hours. Results: When compared to healthy participants (5.8%), acute ischemic stroke patients (9.8%) had considerably lower serum vitamin D levels. Serum vitamin D levels in patients ranged from 5 to 41 ng/ml, with a mean of 19.4 ng/ml. Serum vitamin D concentrations in controls ranged from 6 to 48 ng/ml, with a mean of 30.310.48 ng/ml. Stroke patients (66.7%) had considerably higher rates of vitamin D deficiency and insufficiency than healthy controls (51.9%). Serum vitamin D levels and NIHSS scores at admission and 72 hours later showed a significant connection (p=0.007). Additionally, a significant connection between serum vitamin D levels and mRS scores at discharge and three months later was found (p=0.004). Patients who reported having a major stroke were 11.2 times more likely to have 'insufficient' vitamin D (i.e., deficient and insufficient) (p=0.006). Conclusion: Vitamin D deficiency increases the risk of an acute ischemic stroke and is related to a worse short-term outcome as well as a more severe initial stroke.
Keywords
Vitamin D deficiency increases the risk of an acute ischemic stroke and is related to a worse short-term outcome as well as a more severe initial stroke.
Abbreviations
IS: Ischemic stroke, VD: Vitamin D, 25-OH-D: 25-Hydroxyvitamin D, CT: Computed tomography, MRI: Magnetic Resonance Imaging, NIHSS: National Institute of Health Stroke Scale, mRS: Modified Rankin scale.
Introduction
Over 10%, or 5.7 million deaths per year, are caused by strokes, which are the second biggest cause of mortality globally. Over the next several decades, more cases of stroke are expected to occur . Vigorous control of blood pressure has reduced stroke mortality in affluent nations, but the burden of stroke is still increasing because of an aging population [1, 2]. Furthermore, the rising stroke prevalence in middle income countries is a direct result of longer life expectancies in developing nations [1, 2]. Vitamin D (VD) is an organic substance made up of fat-soluble ecosteroids that is primarily responsible for controlling calcium and phosphorus levels as well as other physiological processes [3, 4].
It has been observed that vitamin D deficiency is a major problem in stroke survivors, with an estimated prevalence of. Vitamin D deficiency has been found as a major problem among stroke survivors, with an estimated frequency of 71% [5]. More than 16,000 black and white patients participated in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, which observed that those who lived in areas with less sunlight exposure had a 56% higher risk of stroke and that those who consumed more dietary sterol had a lower risk of stroke and cognitive impairment [6]. The occurrence of osteoporosis in stroke survivors, the identification of D receptors (VDR) and 1a-OHase activity in the brain, as well as the prevalence of osteoporosis, have all contributed to the possibility of further research into the link between low levels of fat-soluble vitamins and stroke [7, 8] respectively. There may be a connection between vitamin D status and the risk of stroke, according to a number of sizable epidemiological research [9]. To completely comprehend the connection between vitamin D levels and people who have acute ischemic strokes, as well as the possibility that vitamin D may function as a important risk factor, more research is, however, necessary [9-12]. Studies have also shown how serum Vitamin D levels affect the results of stroke in patients. This demonstrates the potential for vitamin D supplementation for secondary stroke prophylaxis, the requirement for additional investigation into vitamin D as a biomarker for cerebral ischemia vulnerability, and the necessity to recognize people at high risk for poor post-stroke outcomes [13].
The purpose of this study was to evaluate vitamin D status in acute ischemic patients and investigate its relationship to the severity of the initial stroke and short-term outcome. Because Bangladesh's average life expectancy at birth is now 72 years, the public health concern for stroke has grown significantly in recent years. The cost of managing stroke patients in low- and middle-income nations is so high that almost all governments cannot afford it, and the resources and budgets allotted cannot cover the cost of treatment. The prevalence of stroke in Bangladesh cannot be estimated because there are no national registers for this illness. However, research has revealed that elderly people typically have greater incidence of stroke. There hasn't been a significant amount of research on the association between low vitamin D levels and stroke in our country. If we explore the relationship between vitamin D and stroke, we should develop an alternative strategy for treating stroke prevention. On the relationship between blood vitamin D levels and stroke in Bangladesh, little information is currently available. Therefore, we would prefer to start assessing the vitamin D status of stroke patients in Bangladesh to check for their relationship so that the study's findings can usher in a new era of research into alternative stroke care and prevention strategies.
Methods
Study design, population and settings
• This study was carried out in the Department of Neurology at Sir Salimullah Medical College & Mitford Hospital, Dhaka, Bangladesh from July 2020 to June 2021. The study included 51 patients with acute ischemic stroke (30 men and 21 women) and 51 control volunteers (27 men and 24 women). Subjects were selected based on inclusion and exclusion criteria. The patient was incorporated after receiving written approval. Aged ≥20years or older, the included patients underwent a neurologist's examination, and acute ischaemia was clinically and magnetic resonance imaging of the brain-evidently identified. Patients with intracerebral hemorrhage, endocrine diseases (thyroid, parathyroid, and adrenal problems), chronic illnesses that would influence bone health corticosteroids, chemotherapy), a history and current risk of debilitating diseases, like malignancies, patients receiving vitamin D supplementation, and pregnant, lactating, and menopausal females and patients taking medications that may affect bone health were all excluded from the study.
• Assessing the initial severity of a stroke using the National Institutes of Health Stroke Scale (NIHSS) both at the time of admission and 72 hours afterwards. There are five score ranges on the scale: 0 for no evidence of a stroke, 1-4 for a small stroke, 5-15 for a moderate stroke, 16-20 for a moderate to severe stroke, and 21-42 for a severe to severe stroke (severe stroke) [14].
• The Modified Rankin Scale (mRS) assessment of the functional result at discharge and three months later. The range is 0 to 6, with 0 representing perfect health with no symptoms and 6 representing death. mRS≤2, indicates a favorable (good) outcome, but mRS≥3 indicates a poor one [15]. The standard laboratory workup included a complete blood count, liver function tests, kidney function tests, fasting blood glucose testing, and electrolytes (including sodium, potassium, calcium, and phosphorus levels) Measure serum vitamin D levels using the enzyme-linked immunosorbent test (ELISA). A centrifuge was used to extract the serum from blood samples. The kit was used to quantitatively measure serum total 25-OH vitamin D3. According to their levels of vitamin D, patients and controls were separated into three subgroups: inadequate (vitamin D levels below 10ng/ml), insufficient (vitamin D levels between 10 and 29ng/ml), and sufficient (vitamin D levels ≥ 30ng/ml) [16].
• The study protocol received approval from the ethical council of the Sir Salimullah Medical College in Dhaka, Bangladesh.
Data collection and laboratory procedures
• Data on the patient's sociodemographics, clinical features, stroke risk factors, and pertinent laboratory tests will be performed and recorded when the patient is included in the study.
• All information was collected and documented, including demographic data (age and gender), serum D levels in the two groups, key characteristics, duration (between the onset of symptoms and enrollment into the study), risk factors for stroke, and pertinent laboratory tests.
• The serum vitamin D levels were calculated using a 95% confidence interval and measured in nanograms per milliliter (ng/mL). Case Report Forms were used to collect and record all of the data (CRF).
Data management and analysis
• To compare two independent variables with parametric data and two dependent variables, respectively, for quantitative data, the independent t test and the paired t test were used. Typically, a one-way analysis of variance (ANOVA) was employed to compare more than two variables. In order to identify the sites with significant differences, a post hoc analysis was carried out to look at possible group combinations. For proportional fluctuations in qualitative data, chi-square was utilized, while Fisher exact was used for variables with small expected numbers. The Pearson correlation coefficient was used to determine whether two variables were correlated. A logistic regression study was done to see if vitamin D's influence on disease state is independent. All statistical analyses were performed using the Statistical Program for Social Science (SPSS), version 25, IBM Corp., Chicago, USA, 2017. P values of 0.05 or higher were regarded as significant, and p values of < 0.01 highly significant.
Definitions
National institute of health stroke scale (NIHSS) [17]
1a: Level of consciousness
Instructions: If a full evaluation is restricted by anything like an endotracheal tube, a language barrier, or orotracheal trauma/bandages, the investigator must make a decision. Only if the patient does not move in response to noxious stimulation (apart from reflexive posturing) is a score of 3 given.
Scale Definition
0 = Alert; keenly responsive.
1 = Not alert; but arousable by minor stimulation to obey, answer, or respond.
2 = Not alert; requires repeated stimulation to attend, or is obtunded and requires strong or painful stimulation to make movements (not stereotyped).
3 = Responds only with reflex motor or autonomic effects, or totally unresponsive, flaccid, and areflexic.
1b: LOC
Questions: The month and his/her age are inquired to patient. There is no partial credit for being close to the answer; it must be accurate. Patients who cannot understand the questions while aphasic or stuporous will receive a score of 2. A 1 is assigned to patients who are unable to speak due to endotracheal intubation, orotracheal trauma, severe dysarthria from any cause, a language barrier, or any other issue that is not related to aphasia. It is crucial that only the first response be scored and that the examiners refrain from giving the patient verbal or nonverbal clues in order to "assist" them.
Scale definition
0 = Answers both questions correctly.
1 = Answers one question correctly.
2 = Answers neither question correctly.
1c: LOC
Questions: The patient is then instructed to squeeze and release the non-paretic hand before opening and closing their eyes. If the hands cannot be utilized, use another one-step command in its place. If a clear attempt is made but fails due to weakness, credit is granted. When a patient doesn't comply with a directive, the task should be performed in pantomime and the outcome graded (i.e., follows none, one, or two commands). One-step commands should be delivered to patients who have physical limitations due to trauma, amputation, or other conditions. The first effort alone receives credit.
Scale definition
0 = Performs both tasks correctly.
1 = Performs one task correctly.
2 = Performs neither task correctly.
Best gaze
There will only be a test of horizontal eye movements. There is no caloric testing done, only scoring of reflexive or voluntary (oculocephalic) eye movements. The score will be 1 if the patient's conjugate ocular deviation is correctable through reflex or deliberate action. Give the patient a 1 if they have isolated CN III, IV, or VI peripheral nerve paresis. In all aphasic patients, gaze is tested. Patients who have experienced eye injuries, bandages, pre-existing blindness, or another abnormality of the visual acuity or fields should be examined using reflexive movements, with the investigator's decision. Sometime the presence of partial gaze palsy can be determined by making eye contact and then moving the patient from side to side.
Scale definition
0 = Normal.
1 = Partial gaze palsy; gaze is abnormal in one or either eyes, but forced deviation or total gaze paresis is not present.
2 = Forced deviation, or total gaze paresis is not overcome by the oculocephalic maneuver.
Visual
Instructions: By using finger counting or a visual threat if necessary, the upper and lower visual fields are assessed. Patients may be reassured, but if they correctly focus on the side of the moving fingers, it is possible to rate this as normal. Visual fields in the remaining eye are assessed in cases of unilateral blindness or enucleation. Score 1 only if an obvious asymmetry is discovered, including quadrantanopia. Score 3 if the patient is blind for whatever reason. At this point, two stimuli are delivered simultaneously. If extinction occurs, the patient receives a 1, and the outcomes are utilized to answer item in the test 11.
Scale definition
0 = No visual loss.
1 = Partial hemianopia.
2 = Complete hemianopia.
3 = Bilateral hemianopia (blind including cortical blindness).
Facial palsy
Instructions: To get the patient to show teeth, raise their eyebrows, or close their eyes, ask them to do so or utilize pantomime. When a patient is inattentive or insufficiently receptive, they will grimace symmetrically in response to unpleasant stimuli. The face should be cleared as much as possible of any physical obstacles, such as orotracheal tubes, tape, facial bandages, and other physical barriers.
Scale definition
0 = Normal symmetrical movements.
1 = Minor paralysis (flattened nasolabial fold, asymmetry on smiling).
2 = Partial paralysis (total or near-total paralysis of lower face).
3 = Complete paralysis of one or both sides (absence of facial movement in the upper and lower face).
Motor arm
Instructions: Put the limb in the proper posture by extending your arms 90 degrees (if you're sitting) or 45 degrees (if you're standing) (if supine). If the arm drops before 10 seconds, drift is scored. Using eagerness in the voice and gesture, but avoiding noxious stimulus, the aphasic patient is urged. Starting with the arm that is not paretic, each limb is tested one at a time. The examiner should only mark the score as untestable (UN) in the event of an amputation or shoulder joint fusion, and they should explicitly explain why in their notes.
Scale Definition
0 = No drift; limb holds 90 (or 45) degrees for full 10 seconds.
1 = Drift; limb holds 90 (or 45) degrees, but drifts down before full 10 seconds; does not hit bed or other support.
2 = Some effort against gravity; limb cannot get to or maintain (if cued) 90 (or 45) degrees, drifts down to bed, but has some effort against gravity.
3 = No effort against gravity; limb falls.
4 = No movement.
UN = Amputation or joint fusion, explain.
Motor leg
Instructions: Place the limb in the proper position by holding the leg at a 30 degree angle (always tested supine). If the leg drops before 5 seconds, drift is scored. Using urgency in the voice and gesture, but avoiding noxious stimulus, the aphasic patient is motivated. Starting with the non-paretic leg, each limb is put to the test one at a time. The examiner shall only mark the score as Untestable (UN) in the event of an amputation or hip joint fusion and explicitly explain this decision.
Scale definition
0 = No drift; leg holds 30-degree position for full 5 seconds.
1 = Drift; leg falls by the end of the 5- second period but does not hit the bed.
2 = Some effort against gravity; leg falls to bed by 5 seconds but has some effort against gravity.
3 = No effort against gravity; leg falls to bed immediately.
4 = No movement.
UN = Amputation or joint fusion, explain:
Limb ataxia
Instructions: The goal of this test is to look for signs of a unilateral cerebellar lesion. Test while keeping your eyes open. Make sure testing is conducted with an undamaged visual field if there is a visual deficiency. Both sides are given the heel-shin and finger-nose-finger tests, and ataxia is only given a score if it is present and out of proportion to weakness. Patients who are paralyzed or unable to understand do not exhibit ataxia. The examiner should only mark the score as Untestable (UN) in cases of amputation or joint fusion and explicitly explain this decision. Test for blindness by having the patient touch their nose with an extended arm.
Scale definition
0 = Absent.
1 = Present in one limb.
2 = Present in two limbs.
UN = Amputation or joint fusion, explain:
Sensory
Instructions: Patients who are obtunded or aphasic may experience a sensation similar to a pinprick when tested, or they may retreat from unpleasant stimuli. The examiner should evaluate as many body parts as necessary, including the arms (not hands), legs, trunk, and face, in order to effectively detect hemisensory loss. Only sensory loss linked to stroke is classified as abnormal. Only when a severe or complete loss of sensation can be demonstrably noted should a score of 2, "severe or entire sensory loss," be assigned. Therefore, individuals who are stuporous or aphasic will likely receive a score of 1 or 0. The brainstem stroke patient is given a score of 2 and has bilateral loss of sensation. Score 2 if the patient is quadriplegic and unresponsive. On this test, patients who are in a coma (item 1a=3) are automatically assigned as 2.
Scale Definition
0 = Normal; no sensory loss.
1 = Mild-to-moderate sensory loss; patient feels pinprick is less sharp or is dull on the affected side; or there is a loss of superficial pain with pinprick, but patient is aware of being touched.
2 = Severe or total sensory loss; patient is not aware of being touched in the face, arm, and leg.
Best language
Instructions: The examination's earlier sections will yield a considerable lot of information concerning comprehension. For this scale item, the patient must name the objects on the accompanying naming sheet, describe what is happening in the accompanying picture, and read from the accompanying list of phrases. Responses to all of the commands in the general neurological exam that came before are used to determine comprehension. Ask the patient to identify objects placed in their hands, repeat them, and make speech if their vision is impaired during the examinations. Asking the intubated patient to write is appropriate. This item will automatically receive a 3 from the patient who is in a coma (item 1a=3). A score of 3 should only be used if the patient is mute and does not follow one-step orders. The examiner must determine a score for the patient with stupor or limited cooperation.
Scale definition
0 = No aphasia; normal.
1 = Mild-to-moderate aphasia; some obvious loss of fluency or facility of comprehension, without significant limitation on ideas expressed or form of expression. Reduction of speech and/or comprehension, however, makes conversation about provided materials difficult or impossible. For example, in conversation about provided materials, examiner can identify picture or naming card content from patient's response.
2 = Severe aphasia; all communication is through fragmentary expression; great need for inference, questioning, and guessing by the listener. Range of information that can be exchanged is limited; listener carries burden of communication. Examiner cannot identify materials provided from patient response.
3 = Mute, global aphasia; no usable speech or auditory comprehension.
Dysarthria
Instructions: Asking the patient to read or repeat words from the pages 6 and 8 of the NIH Stroke Scale paper will provide an appropriate sample of speech if the patient is believed to be normal (pdf, 495kb). The degree of articulation clarity in the patient's spontaneous speech can be assessed if they have severe aphasia. The examiner should only mark the patient's score as untestable (UN) and clearly explain their decision if the patient is intubated or otherwise physically unable to speak. Do not explain the purpose of the test to the patient.
Scale definition
0 = Normal.
1 = Mild-to-moderate dysarthria; patient slurs at least some words and, at worst, can be understood with some difficulty.
2 = Severe dysarthria; patient's speech is so slurred as to be unintelligible in the absence of or out of proportion to any dysphasia, or is mute/anarthric.
UN = Intubated or other physical barrier, explain:
11: Extinction and Inattention (formerly Neglect):
Instructions: During the initial testing, sufficient data might be gathered to determine neglect. The score is normal if the patient has a significant visual impairment that prevents visual double simultaneous stimulation and the cutaneous stimuli are normal. The score is normal if the patient has aphasia but appears to pay attention to both sides. Visual spatial neglect or anosagnosia may likewise be viewed as abnormality-indicating symptoms. The item is never untestable because the abnormality is only assessed when it is present.
Scale definition:
0 = No abnormality.
1 = Visual, tactile, auditory, spatial, or personal inattention, or extinction to bilateral simultaneous stimulation in one of the sensory modalities.
2 = Profound hemi-inattention or extinction to more than one modality; does not recognize own hand or orients to only one side of space
Interpretation of NIHSS scale:
The scale consists of five score sections,
1. Score 0 (no stroke symptoms),
2. Score 1–4 (minor stroke), score 5–15 (moderate stroke),
3. Score 16–20 (moderate to severe stroke), and
4. Score 21–42 (severe stroke)
The Modified Rankin Scale (MRS): The scale runs from 0-6, running from perfect health without symptoms to death.
0 - No symptoms.
1 - No significant disability. Able to carry out all usual activities, despite some symptoms.
2 - Slight disability. Able to look after own affairs without assistance, but unable to carry out all previous activities.
3 - Moderate disability. Requires some help, but able to walk unassisted.
4 - Moderately severe disability. Unable to attend to own bodily needs without assistance, and unable to walk unassisted.
5 - Severe disability. Requires constant nursing care and attention, bedridden, incontinent.
6 - Dead.
Vit D level
According to vitamin D status, patients and controls were subdivided into three subgroups:
• The normal serum reference range for total 25-hydroxyvitamin D is 30–100 ng/mL,
• Vitamin D insufficiency was defined as a serum level <20 ng/mL, and
• Vitamin D deficiency was defined as a serum level <10 ng/mL.
Result
51 patients with acute ischemic stroke (30 male and 21 female) and 51 control subjects (27 male and 24 female) were included in the study. The age of the control individuals ranged from 26 to 70 years old with a mean of 56.34 ± 12.59 years (p=0.613), whereas the age of the patients ranged from 39 to 82 years with a mean of 59.55 ± 10.88 years (Table 1). Among patients and controls 57 (55.9%) were males and 54 (44.1%) were females.
| Age (in year) | Group | p value* | |
|---|---|---|---|
| Case | Control | ||
| ≤50 | 10 (19.6) # | 12 (23.5) | 0.613 |
| 50-59 | 16 (31.4) | 17 (33.3) | |
| 60-69 | 15 (29.4) | 14 (27.5) | |
| >70 | 10(19.6) | 8(15.7) | |
| Total | 51 (100.0) | 51 (100.0) | |
| Mean ± SD | 59.55±10.88 | 56.34±12.59 | |
#Figure within parentheses indicates in percentage.
Table 1. Distribution of age by group.
Forty one patients (80.4%) were hypertensive, 31 patients (60.8 %) were diabetic, 13 patients (25.5%) had ischemic heart disease, 13 patients (25.5%) had atrial fibrillation and 28 patients (54.9%) were smokers (Table 2).
| Response | Number | Percent |
|---|---|---|
| Hypertension | ||
| No | 10 | 19.6 |
| Yes | 41 | 80.4 |
| Diabetes mellitus | ||
| No | 20 | 39.2 |
| Yes | 31 | 60.8 |
| Atrial fibrillation | ||
| No | 38 | 74.5 |
| Yes | 13 | 25.5 |
| Smoker | ||
| No | 23 | 45.1 |
| Yes | 28 | 54.9 |
| Previous stroke | ||
| No | 39 | 76.5 |
| Yes | 12 | 23.5 |
| Previous TIA | ||
| No | 40 | 78.4 |
| Yes | 11 | 21.6 |
| Ischemic heart disease | ||
| No | 38 | 74.5 |
| Yes | 13 | 25.5 |
| Heart failure | ||
| No | 46 | 90.2 |
| Yes | 5 | 9.8 |
Table 2. Risk factors of stroke.
The NIHSS scores on presentation varied from 3 to 29, with a mean of 10.6 ± 5.9. There were 2 patients (3.9%) with severe stroke, 9 (17.6%) with moderate/severe stroke, 31 (60.8%) with moderate stroke, and 9 (17.6%) with mild stroke. After 72hrs, the NIHSS scores ranged from 1 to 25 with a mean of 7.8 ± 5.2. Twelve patients (23.5%) had mild stroke, 34 patients (66.7%) had moderate stroke, 3 patients (5.9%) had moderate/ severe stroke, and 2 patients (3.9%) had severe stroke (Table 3).
| NIHSS score | Number | Mean | Std | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Initial NIHSS score | 51 | 10.6 | 5.9 | 10.0 | 3.0 | 29.0 |
| NIHSS score after 72 h | 51 | 7.8 | 5.2 | 6.0 | 1.0 | 25.0 |
Table 3. Stroke severity on admission and after 72 hrs regarding NIHSS score.
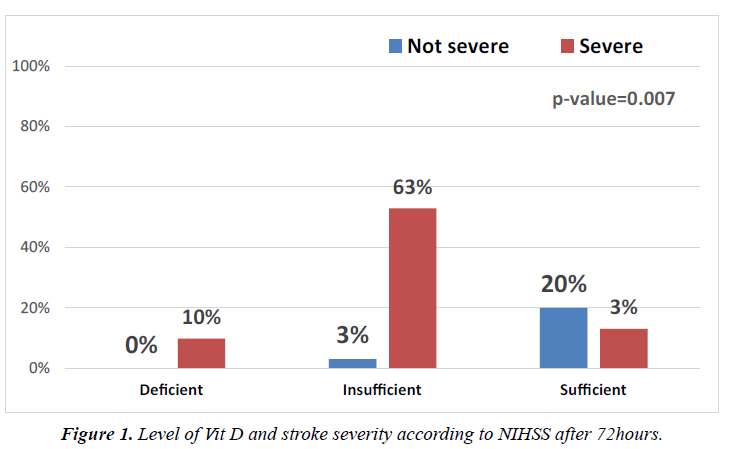
Twelve patients (23.5%) had mild stroke, 34 patients (66.7%) had moderate stroke, 3 patients (5.9%) had moderate/severe stroke, and 2 patients (3.9%) had severe stroke (Figure 1).
Serum vitamin D level: In patients, serum vitamin D level ranged from 5 to 41ng/ml with a mean of 21.4±9.98ng/ml. In controls, serum vitamin D levels ranged from 6 to 48ng/ml with a mean of 30.3 ± 10.48 ng/ml. A statistically significant difference was found between the two groups as regards mean serum vitamin D level, being significantly lower in stroke patients (p value=0.056) (Table 4).
| Subgroup | Number | Mean | Std | 95% confidence interval | p-value | |
|---|---|---|---|---|---|---|
| lower bound | upper bound | |||||
| Control | 51 | 30.3 | 10.48 | 25.4 | 31.2 | 0.056* |
| Patient | 51 | 21.4 | 9.98 | 21.6 | 27.2 | |
Table 4. Comparison of mean serum vitamin D level between study subgroups (ANOVA).
According to vitamin D status, patients and controls were sub-divided into three subgroups: deficient (vitamin D levels <10ng/ml), insufficient (vitamin D levels 10–29ng/ml), and sufficient (vitamin D levels ≥30ng/ ml) 16. A statistically significant difference was also detected between subgroups of patients and controls regarding vitamin D status (p value=0.002) (Table 5).
| Vit D status | Control | Patient | Control | Patient | P value |
|---|---|---|---|---|---|
| Deficient | 3 | 5 | 5.9% | 9.8% | 0.002* |
| Insufficient | 13 | 29 | 25.5% | 56.9% | |
| Sufficient | 35 | 17 | 68.6% | 33.3% | |
| 51 | 51 | 100.0% | 100.0% |
Table 5. Types Vit D status among patient and control.
On comparing Vit D status and NIHSS score between patient subgroups, there was a statistically significant difference detected between patient subgroups regarding initial scores of NIHSS, being significantly higher in the deficient group (p=0.003) (Table 6).
| Vit D status | Number | Initial NIHSS score (mean) | Std | 95% confidence interval | p-value | |
|---|---|---|---|---|---|---|
| lower bound | upper bound | |||||
| Deficient | 5 | 20.4 | 8.96 | 9.3 | 31.5 | 0.003* |
| Insufficient | 29 | 10.9 | 4.06 | 9.4 | 12.4 | |
| Sufficient | 17 | 7.3 | 4.40 | 5.0 | 9.6 | |
* Significant
Table 6. Comparison of vitamin D status and NIHSS scores.
Post hoc analysis revealed a statistically significant difference between deficient and insufficient groups compared to sufficient group (p value=0.01, 0.01 respectively) (Table 7). On discharge, a statistically significant difference was detected between patient subgroups regarding mean scores of mRS, being significantly higher in the deficient.
| NIHSS score | 25 (OH) vit D (ng/ml) |
|---|---|
| NIHSS score on admission | |
| Pearson correlation | -0.508 |
| p-value | <0.01 |
| N | 51 |
| NIHSS score after 72 h | |
| Pearson correlation | -0.509 |
| p-value | <0.01 |
Table 7. Relation between serum vitamin D and stroke severity on presentation and after 72 h by NIHSS.
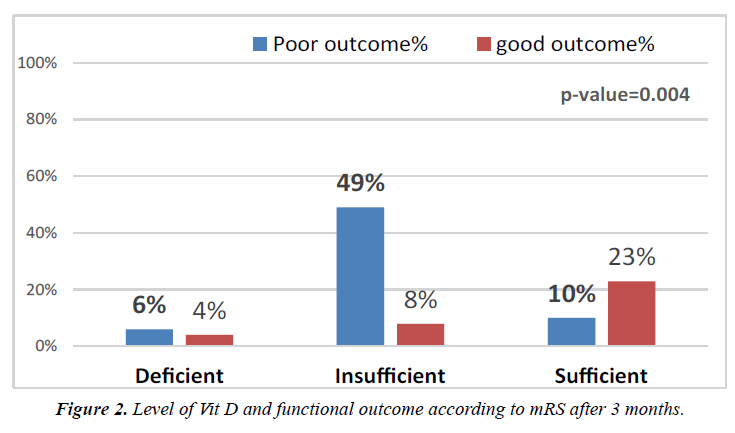
On discharge, mRS scores ranged in deficient from 1 to 5 with a mean of 3.80 ± 1.09 and insufficient was 3.21 ± 0.67. Ten patients (19.6%) had good outcome whereas 41 patients (85.4%) had poor outcome. After 3 months, mRS scores in deficient ranged from 1 to 6 with a mean of 2.60 ± 1.34 and insufficient was 1.97 ± 0.57. Eighteen patients (35.3%) had good outcome; however, 33 patients (64.7%) had poor outcome (Table 8).
| Vit D status | Number | mRS score on discharge | mRS score after 3 months |
|---|---|---|---|
| Deficient | 5 | 3.80 ± 1.09 | 2.60 ± 1.34 |
| Insufficient | 29 | 3.21 ± 0.67 | 1.97 ± 0.57 |
| Sufficient | 17 | 3.06 ± 0.75 | 2.06 ± 0.75 |
| p-value | 0.05 | 0.004 | |
Table 8. Comparison between patient subgroups regarding mean mRS scores on discharge and after 3 months.
On discharge, a statistically significant difference was detected between patient subgroups regarding mean scores of mRS, being significantly higher in the deficient (p=0.05). After 3months, a statistically highly significant difference was detected between patient subgroups regarding mean scores of mRS, being significantly higher in the deficient group (p=0.004).
Eighteen patients (35.3%) had good outcome; however, 33 patients (64.7%) had poor outcome (Figure 2).
A logistic regression analysis was performed to evaluate the independence of vitamin D role in the disease status apart from differences of age, gender distribution, and vascular risk factors. Vitamin D was found contributing to disease status (severity and outcome) independent of age, gender, and vascular risk factors. The Logistic Regression analysis revealed a significant impact of the Vit D status of the patients on the severity of stroke. The patients with 'not sufficient' Vit D (i.e. deficient and insufficient) were 11.2 time more likely to report severe stroke OR=11.2. (Table 9).
| Variable | B | Odds ratio | p-value | |
|---|---|---|---|---|
| Vit D status | Sufficient | - | - | - |
| Not sufficient | 2.42 | 11.2 | 0.006* | |
| Constant | 0.36 | 1.4 | 0.469 | |
| * Significant at less than 1% level of significance | ||||
Table 9. Logistic Regression analysis: Vit D status and severity of stroke.
Discussion
With a decreased incidence of stroke and stroke mortality, vitamin D has the potential to protect against neurovascular injury [16]. Additionally, it has been discovered that low vitamin D levels in stroke patients are closely associated to both a poor functional outcome and an increased risk for future stroke [6]. A statistically significant difference was found in this data, indicating that stroke patients had significantly lower serum vitamin D levels than controls. Furthermore, there was a substantial difference between patient and control subgroups when it came to vitamin D status, with stroke patients significantly more likely to experience vitamin D shortage and insufficiency than control subjects, who typically had adequate vitamin D status. Low levels of 25(OH) vitamin D have been associated with a slight increase in the risk of ischemic stroke in earlier research [7, 8, 14, 15]. The majority of earlier research, which was mostly focused solely on Caucasian populations, showed a sporadic negative correlation between vitamin D levels and the risk of stroke [18]. Recent meta-analyses have provided statistically significant pooled estimates of the relative risks of stroke when comparing individuals with low vs. high vitamin D status and summarized the findings of earlier investigations [19, 20] Similar to this, Brndum-Jacobsen et al. found that the multivariate adjusted OR of ischemic stroke was 1.54 (1.43- 1.65) in a meta-analysis comparing the lowest vs top quartile of 25(OH) D concentration [19].
These simplified findings suggest that low vitamin D deficiency may have a considerable impact on stroke risk. We discovered a high and significant correlation between 25(OH) D3 status and risk of ischemic stroke in the Bangladeshi population, which is consistent with these findings. More severe strokes and poorer post-stroke outcomes have been associated to vitamin D deficiency [21]. Although the exact cause of the association is yet unknown, lower serum levels of 25(OH) D3 are independently linked to larger infarct volumes in stroke patients [16] According to the National Institutes of Health Stroke Scale (NIHSS), patients with 25(OH) D3 insufficiency had poorer stroke severities, whereas those with adequate to optimal VD levels had lower ratings or, on average, less severe strokes [16, 22]. A strong negative correlations between vitamin D level and NIHSS scores beyond 72 hours was discovered in further studies, which supported the persistent relationship between vitamin D and stroke severity. This was in line with earlier studies that had established a causal link between lower 25(OH) vitamin D levels and increased clinical severity [11, 23]. The current study discovered a significant difference in stroke severity between patient subgroups on the basis of a presentation in which declining vitamin D levels were linked to higher NIHSS ratings, which indicated more severe strokes. When patients were discharged, the short-term post-stroke outcome, as determined by the modified Rankin Scale (mRS), also got worse for 25(OH) D3-deficient stroke patients [11, 23] Patients with 25(OH) D3 deficiencies also had higher mRS scores three months after a stroke, indicating particularly severe longer-term outcomes [11, 16, 23]. These studies' findings were supported by our observation that mRS at discharge and three months later confirmed a highly significant difference between patient groupings. Between vitamin D status and mRS at discharge and three months later, a significant negative correlation was additionally found to have changed. Based on a logistic regression study, vitamin D status was found to be independent of illness status, with vitamin D deficiency increasing disease severity by 11.2 times. Similar to Makariou and associates [9] and Zhou, these findings were made [10].
Conclusion
Lack of vitamin D is associated with the early severity of acute ischemic stroke and is a predictor of a poor short-term outcome. These results further support the use of vitamin D supplementation in the secondary prevention of stroke in individuals who already have it and in the primary prevention of stroke in patients with vascular risk factors in order to lessen disability and enhance functional outcomes.
Limitations
Small sample size and this single hospital based study did not reflect exact scenario of the whole community. Patients from all socioeconomic status and all parts of the country did not come to seek medical attention in the study place.
Data Availability
The datasets analysed during the current study are not publicly available due to the continuation of analyses but are available from the corresponding author on reasonable request.
Funding
Funding from Bangabandhu Fellowship under the Ministry of Science and Technology, Bangladesh was received for this study.
Ethical consideration
The study was conducted after approval from the ethical review committee. The confidentiality and anonymity of the study participants were maintained.
Authors’ Contributions
AR was responsible for conception and design, obtaining funds, data interpretation, manuscript drafting and manuscript editing, and final approval data acquisition, data interpretation and critical revision for important intellectual content conception and design, obtaining funds, data interpretation, manuscript editing, and final approval. MNQ was responsible for data analysis and statistical analysis. MJA and AHR were responsible for data collection. All authors have read and approved the final version of the manuscript.
Acknowledgment
The authors were grateful to the staffs and management of the Department (OPD) of Neurology in Sir Salimullah Medical College Mitford Hospital, Bangladesh.
Conflict of Interest
The authors stated that there is no conflict of interest in this study
References
- Kim AS, Johnston SC. Temporal and geographic trends in the global stroke epidemic. Stroke. 2013;44(6):S123-5.
- Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439-48.
- Al Mheid I, Quyyumi AA. Vitamin D and cardiovascular disease: controversy unresolved. J Am Coll Cardiol. 2017;70(1):89-100.
- Alkhatatbeh MJ, Abdul-Razzak KK, Khasawneh LQ, et al. High prevalence of vitamin D deficiency and correlation of serum vitamin D with cardiovascular risk in patients with metabolic syndrome. Metabolic syndrome. 2017;15(5):213-9.
- Gupta A, Prabhakar S, Modi M, et al. Effect of Vitamin D and calcium supplementation on ischaemic stroke outcome: a randomised controlled open‐label trial. Int J Clin Pract Suppl. 2016;70(9):764-70.
- Nair R, Maseeh A. Vitamin D: The “sunshine” vitamin. J Pharmacol Pharmacother. 2012;3(2):118-26.
- Uluduz D, Adil MM, Rahim B, et al. Vitamin D deficiency and osteoporosis in stroke survivors: an analysis of National Health and Nutritional Examination Survey (NHANES). J Vasc Interv Neurol. 2014;7(1):23.
- Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
- E Makariou S, Michel P, S Tzoufi M, et al. Vitamin D and stroke: promise for prevention and better outcome. Curr Vasc Pharmacol. 2014;12(1):117-24.
- Zhou R, Wang M, Huang H, et al. Lower vitamin D status is associated with an increased risk of ischemic stroke: a systematic review and meta-analysis. Nutr. 2018;10(3):277.
- Park KY, Chung PW, Kim YB, et al. Serum vitamin D status as a predictor of prognosis in patients with acute ischemic stroke. Cerebrovasc Dis. 2015;40(1-2):73-80.
- Sun Q, Pan A, Hu FB, et al. 25-Hydroxyvitamin D levels and the risk of stroke: a prospective study and meta-analysis. Stroke. 2012;43(6):1470-7.
- Turetsky A, Goddeau Jr RP, Henninger N. Low serum vitamin D is independently associated with larger lesion volumes after ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24(7):1555-63.
- Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5(7):603-12.
- Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30(8):1538-41.
- Yarlagadda K, Ma N, Doré S. Vitamin D and stroke: effects on incidence, severity, and outcome and the potential benefits of supplementation. Front Neurol. 2020;11:384.
- National Institute of Neurological Disorders and Stroke (US). NIH Stroke Scale: National Institute of Neurological Disorders and Stroke. Dept, of Health and Human Services. USA, 2011.
- Majumdar V, Prabhakar P, Kulkarni GB, et al. Vitamin D status, hypertension and ischemic stroke: a clinical perspective. J Hum Hypertens. 2015;29(11):669-74.
- Brøndum‐Jacobsen P, Nordestgaard BG, Schnohr P, et al. 25‐hydroxyvitamin D and symptomatic ischemic stroke: an original study and meta‐analysis. Ann Neurol. 2013;73(1):38-47.
- Michos ED, Reis JP, Post WS, et al. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: the NHANES-III linked mortality files. Nutr. 2012;28(4):367-71.
- Fahmy E, Sharaf S, Helmy H, et al. Vitamin D status in acute ischemic stroke: relation to initial severity and short-term outcome. Egypt J Neurol Psychiatr Neurosurg. 2019;55(1):1-6.
- Wei ZN, Kuang JG. Vitamin D deficiency in relation to the poor functional outcomes in nondiabetic patients with ischemic stroke. Biosci Rep. 2018;38(2).
- Rezaei O, Ramezani M, Roozbeh M, et al. Does vitamin D administration play a role in outcome of patients with acute ischemic stroke? A randomized controlled trial. Curr J Neurol. 2021;20(1):8.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref