Research Article - International Journal of Pure and Applied Zoology (2022) Volume 10, Issue 6
STRATEGY AND TECHNIQUES USED BY FORAGING SPOT-BILLED PELICAN PELECANUS PHILIPPENSIS IN PULICAT LAKE, ANDHRA PRADESH, INDIA
Vaithianathan Kannan*
PG Research Department of Zoology & Wildlife Biology, AVC College (Autonomous), Mannampandal 609 305, Mayiladuthurai, Tamil Nadu, India
- *Corresponding Author:
- Vaithianathan Kannan
PG Research Department of Zoology & Wildlife Biology
AVC College (Autonomous)
Mannampandal 609 305
Mayiladuthurai
Tamil Nadu
India
E-mail: kannan.vaithianathan@gmail.com
Received: 28-May-2022, Manuscript No. IJPAZ-22-65233; Editor assigned: 31-May-2022, PreQC No. IJPAZ-22-65233(PQ); Reviewed: 14-June-2022, QC No. IJPAZ-22-65233; Revised: 17-June-2022, Manuscript No. IJPAZ-22-65233(R); Published: 24-June-2022, DOI: 10.35841/2320-9585-10.6.126
Abstract
Foraging strategy and feeding techniques of the spot-billed pelican Pelecanus philippensis were studied from October 2010 to September 2012 in Pulicat Lake, Andhra Pradesh. The spot-billed pelicans were observed using visual techniques to catch prey. Of the 1061 observations in 2 years, 72.6% of prey were caught by striking with the wing opened, and 14.1% were caught by driving fish into shallows and 13.3% were caught with the wing closed. The rate of foraging attempts by pelicans fluctuates with season and coincides with prey abundance. During post-monsoon when the water level was high, spot-billed pelican search for prey more often, as the prey became widespread whereas in summer when the water level decreased, the concentration of the prey was lower, which helped the pelicans to catch prey in quick succession. Prey behaviour and habitat condition determine the selection of the visual foraging technique by the pelicans in Pulicat Lake. Pelicans were more successful in the early hours of the day (0600-1000 hrs) and the evening (1400-1800 hrs). Principal component analysis showed that prey handling time, month and water depth determined the foraging success of spot-billed pelicans.
Keywords
Foraging, Techniques, Pelecanus philippensis, Pulicat Lake, Spot-billed Pelican.
Introduction
Foraging refers to the behavior associated with searching, subduing, capturing and consuming food [1,2]. However, it varies with the available habitat from which food is gathered by a species, types of food eaten, and techniques of prey capture of a species [3,4]. Group foraging is common in many animal species (fishes, mammals, birds and insects) and its evolutionary causes have been extensively discussed [5-7]. Group foraging reduced predation risk as an advantage and is associated with a decrease in vigilance rate, which in turn may allow an individual to raise its feeding rate and to decrease the variability over time of its food intake [8-13]. Relatively little attention is given to the effect of prey availability on feeding behaviour and the role of behaviour in prey selection [14]. Every species is adapted to feed most profitably through appropriate feeding mechanisms [15].
Shorebirds are aquatic inhabitants of which the Moorhens occupies quite waters and the Dabbling Ducks are found open waters. The selection of depth of water for feeding is poorly studied and each species have a pattern of behaviour depends on habitats, season food and other local environmental factors. For many avian species, travel to foraging sites can be an important component of an individual’s time and energy budgets, particularly during the breeding season [16]. The cost of travel can be energetically expensive particularly for the large birds employing powered (flapping) flight [17,18]. Like storks, pelicans conserve energy by employing soaring/gliding flight during favourable atmospheric conditions [19]. Foraging flight characteristics, travel strategies and estimated energy costs of travel can provide important information necessary to assess the impact of wetland loss on the successful reproduction of these species.
Exploring the factors influencing food choice by waterbirds are essential to understand their survival needs and adaptations that impose restrictions on how, why and when birds feed. The actions starting from the search for food and intake are termed ‘foraging and that must be efficient as well as effective for the animals to survive. The optimization of effort, time, or energy expenditures in the food search has been termed the ‘optimal foraging theory’, which varies widely so that individuals of the same species do not all feed in the same manner in all areas or at all times. Head and neck swaying are commonly used tactics that permits birds to obtain better parallax [20-26]. Head swaying may help in a precise estimate of distance and location of prey when only a single strike is possible, such as on a particularly cryptic prey or those that can readily escape. Wings are often used in foraging [27-35]. Opening and flicking wings are used to disturb prey and the wing-open posture during feeding help increase visibility by reducing glare. That position is often, although not always assumed with back to the sun.
Pelicans in general, except for the Brown Pelican Pelecanus occidentalis that plunge - dives for their food, are said to have primarily catch prey by forming a flotilla of 8-12 birds, usually in a semi-circle, and driving the fish into shallows [36]. At the end of the drive, they plunge their bills in unison into the water to catch the fish in the front. There could be several variations of this repertoire. Solitary fishing is also reported in some species of pelican [37,38]. The Spot-billed Pelican employs both solitarily and communal feeding methods. Communal feeding in the species can again be divided into compact feeding techniques where the birds group and feed in a concentrated fashion (using the wings-open and strike techniques) and by formations of loose clusters (using the wings-closed and strike technique). The apparent casualness of the wings closed and strikes feeding technique, suggests that the birds are fishing on slow prey, possibly prawns or fish fry. Small fish forms a good part of the diet of many pelican species [39,40]. Another feature of foraging strategy is the coordinated group fishing established by the Great White Pelican where each bird hunts on its own where fish are abundant [41]. Studies on the Great White Pelican showed that solitary birds and individuals in non-synchronized flocks had a markedly higher prey capture rate than those in synchronized flocks and that prey capture rate declined with increasing flock size [42,43]. However, capture success in trapping large, mobile fish is hypothesized to be higher in flocking birds, which explains the significance of the energetic wings open and strikes strategy observed in the Spot-billed Pelican in Pulicat Lake [44]. The relationship between morphology and feeding behaviour suggests limits on the choice of behaviours that can be made by various species. Hence I asked the following questions: Do the pelicans use special behaviours to enhance their foraging in the specific habitat? Is there any indication of other specific behaviour changes? Is there any spatial segregation between different species of birds or between different individuals of the same species? In a five-minute observation, how many times does the feeding behaviour change?
Methods
Study area
The study was confined to Pulicat Lake (Figure 1) (13° 33’ 34.19” N 80° 10’ 17.40” E) the second largest brackish water lake after Chilika, (Orissa) in India covering an area of 720 km2. Pulicat Lake is one of the most important refuge for waterbirds in southern India and identified as an IBA site by BirdLife International and Bombay Natural History Society and is also proposed for inclusion as a Ramsar Site of Wetland of International Importance by Wetlands International [45,46]. However, the Pulicat Lake is made up of mudflats (60%), tidal flats (10%), openwater (20%), river mouth (5%) and freshwater (5%).
Pelicans are amongst the most distinctive of birds and thus are instantly recognizable [47]. The family Pelecanidae comprises of only one genus, Pelecanus and at present, eight species [48]. The strongholds of the species are in India, largely distributed and confined to southern and north-eastern India, Sri Lanka, Cambodia, Sumatra, Thailand, Philippines [49-59]. Historically the species was reported in Java, Pakistan, Bangladesh, Indonesia, Nepal, Turkey, Laos, Malaysia, Korea, Thailand, China, Vietnam and the Philippines [60-67]. Due to the decline over time and much-reduced distribution range besides other factors the Species Survival Commission (SSC) and the Pelican Specialist Group have strongly urged for studying the species in depth in India [68-73]. The species is notified as Near Threatened [74].
Studies were conducted from October 2010 to September 2012 at Pulicat Lake. Observations on the Spot-billed Pelican in the lake were made using binoculars; spotting scope and a digital camera were used to record the specific events of foraging activity. Five-minute observations were made to record the starting and ending time of each foraging bout, number of foraging attempts, number of foraging success, water depth, habitat type, feeding strategy and mode of prey capture. Water depth was assessed by comparing the tarsus length of large wading birds such as painted storks and egrets in the vicinity. From the general activity pattern records, the total time spent was calculated. During these observations, the numbers of successful/unsuccessful foraging attempts and feeding behaviours were recorded. When a foraging flock was located, it is observed for 5-minutes and after that observation was moved to another active forager. Thus, during the whole study 1061 foraging sequences were observed. The percentage of the total number of times the birds were recorded in each foraging technique was used to estimate the category frequencies. Multivariate analyses were performed and correlation coefficients were calculated based on differences in frequencies of use of foraging strategies. Foraging habitat was defined as mudflats, tidal flats, open water, river mouth and freshwater. Mann-Whitney U-tests were used to test the differences in the rate of foraging attempts recorded in different habitat types in different months by pelicans. One-way ANOVA was used to compare the mean intervals between the associated species such as Painted Storks and Egrets. Principal Component Analysis (PCA) was applied to the foraging variables of pelicans and other associated species to find out which factors determine the role in the foraging success of Spot-billed Pelican feeding success in various habitat types of Pulicat Lake.
Results
Foraging strategy
The following three types of foraging techniques used by the Spot-billed Pelican in Pulicat Lake were recorded.
Wings opened and strikes: As the prey is sighted, the pelicans in the front of the flock stop swimming, sometimes forming a semi-circle, and with partially opened wings, extend their necks and thrust their bills into the water in unison and other birds of the flock repeat the same process a little away. In this foraging technique, the birds may move about rapidly hunting fish, or even leisurely, appearing to just probe the bills into the water to strike upon chance. Solitary birds were also observed to use this technique, but here the strikes appeared to be intentional on sighting fish.
Wing closed and strikes: While swimming the neck is held erect with only the bill pointing down. The bird appears alert presumably looking for fish. On spotting the prey, it dips or strikes the bill into the water quite leisurely, keeping the wings closed during the whole operation. Solitary birds and those feeding in loose flocks mainly use this feeding technique, and this technique was observed even in relatively deep water.
Drive the fish into shallow: In this strategy, the pelicans swim against the water current and quickly move towards the shallow area, driving and catching the prey. The wings are kept closed if the fish is caught at the edge at the end of the drive. If the fish is in deeper water, they generally thrust for fish with partially opened wings. This technique is largely seen in flocks, but at times adopted by solitary birds, and was mostly seen around culverts.
Foraging techniques rates of Spot-billed Pelican
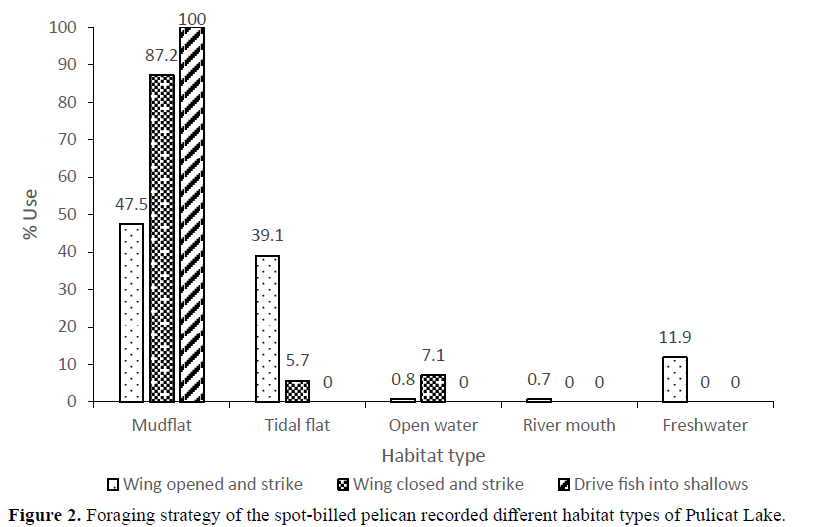
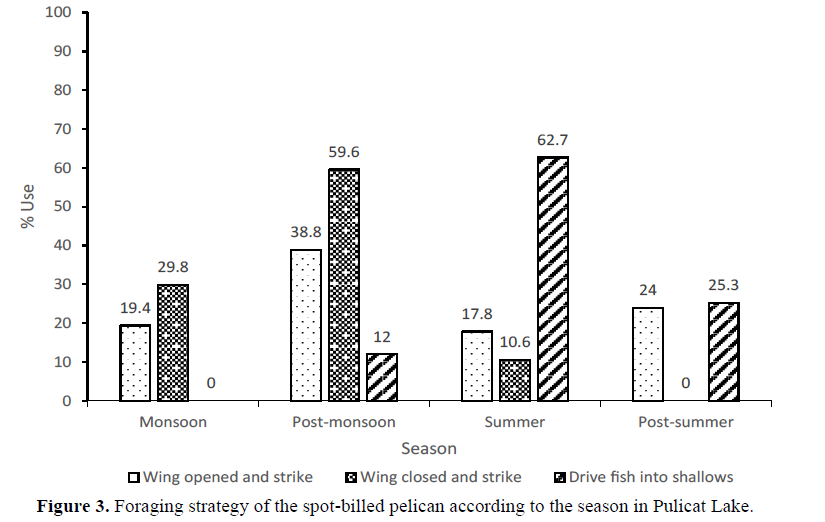
Of the 1061 independent 5-min observations done on Spot-billed Pelicans’ feeding activity in the study period (2010-2012), prey capturer by “wings opened and strikes” mode of feeding was 770 (72.6%) followed by “driving fish into shallows” 150 (14.1%) and “wings closed and strike” mode of foraging was 141 (13.3%). All these strategies used by pelicans were of visual foraging and no tactile foraging was observed, however, casual dipping of bill in the water is observed. The feeding strategy that drives fish into shallows also involved a combination of wing opened and strikes. Across different habitat types, the wings opened and strike strategy in freshwater, mudflat, open water and tidal flats, whereas the wings closed and strike strategy was highest in river mouth (Figure 2) This could be due to the running water and the fishes passing from the rivers into the lake. The rates of foraging techniques of the spot-billed pelican differed significantly in April 2010 (5.6±1.0 number of attempts in 5 min, U=1.258, P<0.084) from that in other months. The attempt rates were more during June (22.3±10.8) and July (20.8±14.5). There was no significant difference among months during 2010-2011. The foraging success of the spot-billed pelican was high in June 2010 (3.6±2.7 number of prey caught in 5 min). The foraging success rate among months differed significantly during April 2.6±0.7 (U= 1.740, P<0.005), May 0.7±0.8 (U=1.934, P<0.001), June (3.6±2.7) (U=1.394, P<0.041), August 1.5±1.3 (U=1.328, P< 0.059), January 1.8±1.5 (U=1.745, P<0.005), February 1.9±1.7 (U=1.288, P<0.073) and March 2.2±1.7 (U=1.358, P<0.050) (Figure 3).
In 2011-2012, the trend was slightly different when compared with 2010-2011. In August 2011 the foraging attempt rate (2.1±1.9) (U=1.668, P<0.008) of the spot-billed pelican differed significantly. The attempt rates were more during April (5.4 ±2.4), May (5.4±3.1) and June (11.0±6.4) (number of attempts in 5 min).In addition, there is no significant difference in the foraging rate of pelicans during 2011-2012 among months. Similarly to 2010-2011, in June 2011-2012 the foraging success was high (3.0±2.1) (number of prey caught in 5 min). The foraging success rate of pelicans differed significantly during April (2.6±0.7) (U=1.581, P<0.031), August (0.7±0.8) (U=1.652, P<0.009), September (0.7±.0.7) (U=1.254, P<0.086), October (0.8±0.8) (U=1.650, P<0.009), November (0.8±0.7) (U=1.266, P<0.081), December (0.8±0.7) (U=1.266, P<0.081), January (0.7±0.7) (U=1.493, P<0.023), February (0.8±0.8) (U=1.650, P<0.009) and March (0.7±0.7) (U=1.493, P<0.023). The difference in the foraging rate of pelicans could be due to the new requirements that feed on the fast depletion of resources or due to intra-species competition.
Foraging success of spot-billed pelican across months and water levels
From the general feeding activity patterns, it was observed that the pelicans were successful in the early hours of the day (06:00- 10:00). The foraging activity declined from 1000 hrs to 1500 hrs. Again after 1500 hrs, the pelicans were actively foraged until 1800 hrs. Habitat wise analysis showed that in 2010-2011, the feeding success was high in freshwater (2.5±2.4), followed by open water (2.1±1.9) and in river mouth (2.0±1.8 number of success in 5 min). In 2011-2012, the feeding success was high in freshwater (1.0±0.7) and mudflat (0.9±1.1 number of success in 5 min) (Table 1). Of the 1061 independent observations on foraging pelicans in two years, almost 100% were through visual mode. Pelicans were able to catch more prey when the water level was less than 50 cm. The highest success in foraging was in 30 cm depth (25.7%), followed by 40cm depth (24.2%) indicating that pelicans require less than 50 cm of water depth for successful foraging (Table 2).
| Habitat | Year | N | Water depth | Flock size | No of feeding attempt | No of feeding success |
|---|---|---|---|---|---|---|
| Freshwater | 2010-2011 | 92 | 19.0±6.5 | 25.0±8.8 | 11.4±10.8 | 25.0±2.4 |
| 2011-2012 | 48 | 6.2±3.7 | 26.6±13.7 | 4.8±3.2 | 1.0±0.7 | |
| Mudflat | 2010-2011 | 215 | 10.0±7.1 | 37.5±11.3 | 10.2±10.4 | 1.9±1.8 |
| 2011-2012 | 209 | 8.1±3.5 | 32.8±12.8 | 4.6±3.5 | 0.9±1.1 | |
| Open water | 2010-2011 | 46 | 39.2±14.1 | 23.2±6.6 | 17.7±9.5 | 2.1±1.9 |
| 2011-2012 | 22 | 9.7±2.6 | 25.2±13.3 | 2.6±2.4 | 0.5±0.5 | |
| Tidal flat | 2010-2011 | 74 | 8.7±4.9 | 27.8±13.0 | 7.1±4.5 | 1.7±1.6 |
| 2011-2012 | 33 | 7.7±3.4 | 40.0±8.5 | 3.2±2.3 | 0.6±0.7 | |
| River mouth | 2010-2011 | 71 | 11.8±5.2 | 41.1±8.8 | 8.4±5.5 | 2.0±1.8 |
| 2011-2012 | 10 | 7.4±5.6 | 36.0±18.0 | 4.4±4.1 | 0.5±0.7 |
Table 1. Feeding success rate of the spot-billed pelican in different habitat types in Pulicat Lake.
| Water level (cm) | No of observation | No of attempt | No of success |
|---|---|---|---|
| 15 | 197 | 6.6±6.2 | 1.3±1.4 |
| 20 | 36 | 21.5±6.9 | 1.8±1.8 |
| 30 | 211 | 5.8±4.5 | 1.3±1.3 |
| 40 | 198 | 10.0±9.8 | 2.3±2.3 |
| 50 | 178 | 7.3±9.1 | 1.3±1.3 |
Table 2. Number of observations (duration 5 min) on spot-billed pelican foraging success and water level (mean number of successful attempts per 5 min±SD).
Foraging attempt rates of painted storks
Of the 514 independent 5-min observation on painted storks in 2 years, 402 (78.2%) of prey capture was through tactile mode of feeding and the remaining 112 (21.8%) were caught by visual mode of feeding. In 2010-2011, of the 216 prey caught 176 (79.6%) were caught through the tactile mode and the remaining 45 (20.3%) through the visual mode. In 2011-2012, of the 307 prey caught, 220 (75%) were caught by the tactile mode of feeding and the remaining 73 (24.9%) were caught by the visual mode of feeding. The foraging attempt rates of the painted storks did not differ significantly over the years. However, in 2010- 2011 the success rate of the painted storks differed significantly during May (0.8±0.6 number of prey caught in 5 min) (U=1.520, P<0.020), July (0.8±0.6) (U=1.682, P<0.007), December (0.3±0.4) (U=1.368, P<0.047), January (0.6±0.7) (U=1.615, P<0.011) and February (0.7±0.6) (U=1.234, P<0.095). In 2011-2012 the painted storks caught significantly during April (0.9±1.4) (U=1.907, P<0.001), May (1.3±1.8) (U=1.916, P<0.001), June (0.9±1.4) (U=2.017, P<0.001), August (1.1±1.4) (U=1.789, P<0.003), December (1.0±1.4) (U=2.008, P<0.001), March (1.0±1.6) (U=1.663, P<0.008). The difference between the two years could be due to the late breeding of painted storks in a nearby heronry (Beripeta) during 2011-2012.
The flock size of the painted storks was more in the freshwater (25.7±19.6), mudflat (30.9±15.9) and tidal flat (19.5±19.0). The foraging success rate was more in mudflat (1.2±1.3) and river mouth (1.2±1.9), similarly the foraging attempt rate was also more in mudflat (5.4±2.6), river mouth (5.3±2.6). The painted storks can feed >50 cm depth water where the pelicans were less successful in feeding and the egrets cannot stand. However, the storks were more successful in 20 cm (1.1±1.5 number of prey caught in 5 min) and 40 cm (1.2±1.6 number of prey caught in 5 min) depth. The flock size of the painted storks was more in the freshwater (25.7±19.6),then the mudflat (30.9±15.9) and then the tidal flats (19.5±19.0). The foraging success rate was more in mudflat (1.2±1.3) and river mouth (1.2±1.9), similarly the foraging attempt rate was also more in mudflat (54.0±2.6), river mouth (5.3±2.6). The painted storks can able to feed >50 cm depth water where pelicans and egrets cannot successfully feed. However, the storks were more successful in 20 cm (1.1±1.5 number of prey caught in 5 min) and 40 cm (1.2±1.6 number of prey caught in 5 min) depth (Table 3).
|
Water level (cm) |
No of observation | No of attempt | No of success |
|---|---|---|---|
| 15 | 119 | 3.2±3.0 | 0.9±1.2 |
| 20 | 70 | 5.0±2.7 | 1.1±1.5 |
| 30 | 140 | 4.5±2.7 | 0.8±1.1 |
| 40 | 93 | 4.2±2.9 | 1.2±1.6 |
| 50 | 92 | 4.7±2.7 | 0.9±1.2 |
Table 3. Number of observations (duration 5 min) on painted stork foraging success and water level (mean number of successful attempts per 5 min±SD).
Foraging attempt rates of egrets
Of the 469 independent 5-min observations on egrets in 2 years, 367 (78.3%) of prey capture by visual mode of feeding (wait and strikes), and the remaining 102 (21.7%) were caught by non-visual mode of feeding. In 2010-2011, of the 392 prey caught 153 (72.9%) were caught by the visual mode of feeding and the remaining 57(27.1%) were caught by the tactile mode of feeding. In 2011-2012, of the 473 prey caught, 176 (68.0%) were caught by the visual mode of feeding and the remaining 83 (32.0%) were caught by tactile mode of feeding. The foraging attempt rates of the egrets did not differ significantly during 2010-2011. However, during 2011-2012 in September the attempt rates differed significantly 5.7±4.4 (number of attempts in 5 min) (U=1.418, P<0.036). During 2010-2011 the success rates differed significantly during April (1.0±0.7) (number of prey caught in 5 min) egrets caught significantly (U=1.292, P<0.071) and May (0.6±0.7) (U=1.380, P<0.044). The flock size of the egrets was more in the open water (69.8±12.1), freshwater (36.6±10.3) and tidal flat (34.1±14.0). The foraging success rate was more in mudflat (2.9±2.6), river mouth (2.2±1.8) and in open water (2.0±1.9) whereas the foraging attempt rate was high in mudflat (12.8±12.6) and tidal flat (10.0±9.4) (Table 4). The reason for the high feeding success rate of egrets could be due to its overcrowding at the foraging site which helps the birds to fly and disturb the prey enhancing the feeding efficiency of the egrets in the open water habitat. Unlike the pelicans and storks, the egrets can be able to feed less than 50 cm of water depth. However, the attempt rates were more in the deep waters. The egrets were more successful in less water depth, which uses other techniques while feeding in a combination of search, strikes, wait and wing-flashing behaviours.
|
Water level (cm) |
No of observation | No of attempt | No of success |
|---|---|---|---|
| 2 | 4 | 5.7±2.2 | 1.3±0.9 |
| 4 | 24 | 9.3±8.4 | 1.9±1.2 |
| 6 | 41 | 4.8±3.3 | 1.2±1.1 |
| 10 | 72 | 5.1±2.4 | 0.9±0.7 |
| 12 | 34 | 7.1±5.4 | 3.0±2.5 |
| 15 | 50 | 20.7±11.5 | 2.1±1.9 |
| 50 | 10 | 31.0±6.0 | 7.6±2.0 |
Table 4. Number of observations (duration 5 min) in relation to egrets foraging success and water level (mean number of successful attempts per 5 min±SD).
Foraging success among the associated species the success rate was more in pelicans and egrets than the painted storks. The highest foraging success for pelicans was in freshwater (2.0±2.1), river mouth (1.9±1.8) and open water (1.6±1.7). A similar success rate was observed in the mudflat and tidal flat. For the storks, the highest success rate was in open water (1.2±0.6) and mudflat (1.1±1.5). For the egrets, the highest success rate was in mudflat (2.3±2.2), river mouth (2.2±1.0), and open water (2.0±1.9) (Table 5). The principal component analysis of the egrets accounted for 20.7% of the total variance. All the values were positive except for the flock size. The highest correlation was with water depth followed by foraging success and foraging attempt. Thus, the high values on the egrets correspond to the foraging success of egrets with water depth followed by the number of foraging success (dependent variable) and the number of foraging attempts in every foraging bout. The water depth determines the number of foraging success of the storks accounted for 15.7% of the total variance, and all the values were positive (Table 6). In painted storks, the highest correlation was with water depth followed by flock size, foraging success. This high values for the storks correspond to the foraging success of storks with water depth followed by the number of foraging successes of the storks. However, the foraging success was negatively correlated with that of water depth and a thick flock of birds that determines the number of foraging success of the pelican. There was a significant correlation (P<0.01) between the flock size of the egrets and pelicans. Thus, the numerically abundant storks reduce the foraging success of the egrets and pelicans (Table 7). On the other hand, the egrets are unable to forage in the deep waters, which are frequented by the pelicans and storks.
|
Habitat |
Spot-billed Pelican | Painted Stork | Egrets |
|---|---|---|---|
| Feeding success | Feeding success | Feeding success | |
| Freshwater | 2.0±2.1 (n=140) | 0.8±1.0 (n=97) | 1.2±1.3 (n=114) |
| Mudflat | 1.4±1.6 (n=424) | 1.1±1.5 (n=247) | 2.3±2.2 (n=175) |
| Open water | 1.6±1.7 (n=68) | 1.2±0.6 (n=10) | 2.0±1.9 (n=7) |
| Tidal flat | 1.4±1.5 (n=107) | 0.9±1.1 (n=86) | 1.5±1.6 (n=130) |
| River mouth | 1.9±1.8 (n=81) | 0.8±1.3 (n=74) | 2.2±1.9 (n=42) |
Table 5. Comparative foraging success rate (number of prey caught in 5 min) of spot-billed pelican, painted storks and egrets in various habitat types of Pulicat Lake.
|
|
Components | ||
|---|---|---|---|
| Spot-billed Pelican | Painted Stork | Egrets | |
| % of total variance accounted for | 12.4 | 15.7 | 20.7 |
| Cumulative % of total variance accounted for | 48.9 | 36.4 | 20.7 |
| Water depth (cm) | 0.284 | 0.297 | 1.000 |
| Flock size | 0.077 | 0.035 | -0.220 |
| No of foraging attempt | 0.053 | 0.014 | 0.083 |
| No of foraging success | -0.070 | 0.020 | 0.214 |
Table 6. Summary of the results of principal component analysis of each of four variables that determined the foraging success of the pelicans, storks and egrets.
|
Variables |
Pelicans (n=820) | Storks (n=514) | Egrets (n=469) |
|
Water depth (cm) |
1.000 (n.s) | 1.000 (n.s) | 1.000 (n.s) |
|
Flock size |
-0.251** | 0.161** | -0.359** |
|
No of foraging attempt |
0.320** | 0.125** | 0.135** |
|
No of foraging success |
0.213** | 0.006 | 0.197** |
Table 7. Correlation matrix(r) for four variables which determined the foraging success of pelicans, storks and egrets in Pulicat Lake (**P<0.01).
Discussion
Spot-billed Pelican uses the visual mode of feeding in Pulicat Lake. Their strategies and techniques have not been described previously. Almost 100% of the foraging attempts of pelicans were of the visual mode of foraging. During summer, the Pulicat Lake dries up, though the pelicans resorted to visual foraging. Furthermore, the pelicans preferred to feed in the places where the water level was generally shallower (less than 50 cm). Other birds feeding along with pelicans use tactile and visual modes of feeding. Wood storks and ibises generally use tactile foraging technique when clear water and high prey density is available [75]. The water level contributes to the abundance of prey in Pulicat Lake. Other wetlands are seasonal and depending on the monsoon. Rainfall is likely to influence many aquatic birds, especially when it is both highly seasonal and variable [76].
There is a general correlation in wading birds between their size and feeding activity [77]. A bird is more likely to choose behaviour based on its success rate or the time between successes than on net energy return. Similarly, among various species, it is expected that the species that are more successful in using a particular behaviour will use it more often than a species that has been not successful with the same behaviour Morphological and behavioural adaptations aid in greater success with specific behavior [78]. An individual may switch behaviour because of changes in the pattern of prey availability or various other external factors [79,80]. When the oxygen levels become less restrictive during the day light hours in the waters, fish may become less available near the surface and have more active feeding behaviours are to be adopted by the foraging birds. Hence, the prey availability may correlate with the selection of particular feeding behaviours. Different habitats or structural niches may require different feeding strategies and hence behaviours vary between habitats and seasons and time.
Foraging in the presence of competitors is probably used to fast depletion of resources or enhancing feeding success. Central place foragers may show their foraging to monitor the activities of potentially aggressive competitors. When we take into account of this hypothesis, most wading birds maximize their food intake at dawn or dusk and are less active during midday. The success rate of feeding does not necessarily increase because of commensal feeding. The success of an individual strike depends on the prey species and the nature of the habitat [81]. The reason could be due to the large flat area where the diversity of prey species is higher. Spatial segregation of foraging areas results in resource segregation because small fish congregate in shallow areas, whereas larger fish was restricted to deeper waters.
Ultimately the success of an individual feeding technique depends on the prey species and nature of the habitat, and this appears to be true in Pulicat Lake as well. However, when a large number of fish-eating birds gather the fish are distributed and the wading birds catch the fleeing fish after short chases. Wading birds select the most appropriate foraging behaviour for their needs, and the choice of successful foraging behaviour should reinforce repeated use. Therefore, the selection of a particular foraging technique among fish-eating birds depends on the condition of the wetland, prey behaviour and the quality of the wetland. The wetland condition determines whether foraging birds should remain as a specialist or generalist. Spotbilled Pelicans used visual foraging techniques in Pulicat Lake due to the shallow and rapidly changing water levels. When the water level decreased, the turbidity increased, especially in summer. In the rainy season, water was less turbid and fishes were more available. Pelicans usually go for prawns even though fishes were present in large numbers. Thus, Spot-billed Pelicans tend to be opportunists to the changes in the Pulicat Lake. Prey selection of birds that mostly search for prey should be generalists, while birds that actively pursue particular prey and those that wait for the prey to approach them should have more restricted diets. Spot-billed Pelicans being a visual predator should have a generalized diet, especially in prey, it can most easily catch. This depends on the type of various fish species and prey items, such as surface feeders, bottom dwellers, etc. The reason why Spot-billed Pelicans select other prey species in bigger wetlands like Pulicat Lake might be due to the competition.
Fishing behaviour of birds was related to the depth at which the birds were able to forage and the presence or absence of associated birds. In Pulicat Lake, the water level was unstable and is deeper in the southern (lagoon) part. The pelicans avoided such areas, as they could not successfully prey. Foraging success of a particular wading bird species also may rely on specific aspects of water level fluctuations, such as depth or concentration and entrapment of prey through water level recession [82]. When pelicans foraged at the fringes, the foraging attempt rate decreased. Higher water levels (>50 cm) are not suitable for wading birds whereas, pelicans use this patch and are free from competition and have more prey species. The spatial segregation of the foraging area results in resource segregation because small fish congregated in shallow areas, whereas larger fish were restricted to deeper waters. In ambient air moisture deficit and high temperature in Pulicat Lake may result in unstable water levels and high dissolved solids. In response to the fluctuating water, regime fishes and other aquatic biotic communities adjusting to changing water levels. During the summer at Pulicat Lake, the shallow feeding zones dry up and were supplied with only small volumes of water from windgenerated tides. As a result, seasonal wetlands such as Kudiri, Koridi and Mallam tanks were used during April, May and June by the birds. However, pelicans use these seasonal wetlands for a shorter period, which again drives the birds back to Pulicat Lake during the drawdown phase. The amount of available water within the lake was reduced and resulted in a change in the prey items consumed by pelicans, whereas, the prey items in the diet of pelicans vary during winter as snails, freshwater fishes, frogs, tadpoles are eaten. During average precipitation, pelicans largely shift to seasonal water bodies surrounding Pulicat Lake. Water conditions, in turn, reflect major changes in the abundance and availability of prey items that are produced in temporary, seasonal and semi-permanent wetlands. Birds responding to this unpredictable potential can compensate for the periods of drought or scanty rainfall through expansion during ideal conditions [83]. Evidence of changing water conditions in waterfowl response was presented by [84-88]. Spot-billed Pelican distribution and its associated major feeding birds at Pulicat Lake are closely related to yearly variations depending on seasonal water bodies.
Distribution of breeding waterfowl on the prairie pothole region is closely related to yearly variations in the frequency of seasonal ponds [89]. Seasonally flooded water bodies surrounding Pulicat Lake dry during summer. During the years of scanty precipitation and run-off factors influencing the water regime, include basin morphology, size and conditions of the drainage basin, ground-water, precipitation, and evaporation rations [90,91]. Kudiri tank support pelicans and prey availability that is adapted for only a limited period. Fishes and other dietary items are abundant during the pelican-breeding season, and because of the shallow water, depths are available to pelicans and their associated co-generic species.
Observations on the sightings of foraging pelicans and the extent or availability of habitat types, it appears that pelicans avoid the relatively deep open water, primarily found in the southern (lagoon) part of Pulicat Lake. The few sightings of pelicans in open water were of birds near the shore. Other studies have shown that pelicans prefer to forage near the shore in large wetlands [92,93]. Pelicans foraged mostly in mudflats, tidal flats, river mouths and freshwater habitats. Open water with more than 60 cm was not found suitable for feeding pelicans. However, pelicans use this habitat during summer or when the water level recedes. Another important feeding strategy was around the culverts elsewhere in Pulicat Lake. Pelicans concentrate in this area as the flow of water through the culverts result in the concentration of fish these sites are prime sites for laying fish traps during the monsoon season. The presence of culverts also helps pelicans to use the foraging strategy of driving fish into shallows. Capitalizing of road culverts for fishing has been reported in the American White Pelican [94]. Culverts become more attractive to pelicans during the drying stages due to the congregation of fish in pools adjoining them where available. In general, pelicans prefer areas of alternating shallow and deep water formed by trenches, culverts, shallow pits and bay-like formations for foraging. This is because pelicans mostly forage in the upper water column and concentrate fish in the shallows or restrict the movement within a given area, and the conditions described above would facilitate these foraging strategies. The depth of the water also determines the availability of bottom-dwelling fish species and prawns to pelicans. Pelicans avoid wetlands with dense aquatic vegetation. However, in some wetlands adjacent to Pulicat Lake, pelicans were found feeding in the thickly vegetated streams during the drying stages. This is in contrast with the Pink-backed Pelican, which prefers weedgrown lagoons to open waters.
Seasonal wetlands due to abundant prey provide ideal sites for storks and egrets results reduce inter-specific strife on pelicans. Seasonal wetlands are composed of shallow low marsh zones. Likely, similar zones in Pulicat Lake will also not contain the surface during summer when seasonal wetlands are prematurely dry. The loss of shallow feeding zones in Pulicat Lake may be more important factors to pelicans feeding ecology during the dry season than the loss of seasonal water areas per se. However, the drawdown appears to compensate for the loss of seasonal wetlands. These are an important element during summer. The short-term increase in prey availability to pelicans may result due to the shallow water conditions and the concentrations of prey items by reduced water volume. In the end, if the complete drawdown occurs, the prey base is eliminated or greatly reduced and food conditions for pelicans rapidly deteriorate.
Falling water levels permit the cycle to complete and provide the conditions that support high prey density following a subsequent rise in water levels. The effect of water level fluctuations can be detrimental to the prey base that is utilized by pelicans if they are of short duration. Water level fluctuations reportedly affected waterfowl and invertebrates [95]. However, largesized fishes associated with permanent water cannot adjust to short-term drawdown that exposes and inundate the mudflats. When the water level rises and drowns the prey, items become abundant. The moisture deficit associated with the semi-arid climate is an integral part of the ecology of the Pulicat Lake and pelicans. Pelican response: Pulicat Lake may provide a variety of items available to pelicans during the years when rain. Under these conditions, different prey items are consumed due to different prey available. The change in bird use was highly concentrated with a reduction in available surface water from April to September. Food availability as a timing factor in the sexual cycle of birds [96].
Conclusion
Summary statistics show that pelicans, storks and egrets have a similar niche breadth in Pulicat Lake. Storks and egrets often shift to temporary wetlands, crop fields and freshwater tanks. The smaller niche breadth was due to the smaller number of prey species consumed and the dominance of mullets (fish) in the diet. Prey taken by the pelicans in Pulicat Lake was mostly of brackish water fishes and crustaceans whereas storks and egrets in the Pulicat Lake were more diverse, including both freshwater fishes, snails, mussels and brackish water prey as well as both aquatic and terrestrial insects and crustaceans. Storks and egrets feeding in freshwater marshes, tanks were at the edge of ponds, whereas pelicans foraged in Pulicat Lake, freshwater tanks and tidal flats and open water. Thus, the greater niche breadth in Pulicat Lake may be associated with a greater diversity of prey available in each habitat. However, pelicans feed in drying ponds and stagnant water bodies in Pulicat Lake. The food niche breadth of pelicans is greater with diverse fish species and crustaceans and is different from storks and egrets. Storks and egrets are generalists, which are feeding on whatever is most available and using diverse nesting and resting sites, results and have been able to fit into a variety of available habitats, according to dynamic conditions. Egrets use foot stirring to disturb or attract prey. Response of birds to fish span is an opportunistic behaviour demonstrating a wide variety of dietary needs and flexibility in changing conditions.
Acknowledgement
I thank the Andhra Pradesh Forest Department and Pulicat- Nelapattu Forest Department staff for the study permission and co-operation during the study. I want to thank Dr. R. Nagarajan (Principal, AVC College (Autonomous)/Head of Postgraduate & Research Department of Zoology & Wildlife Biology) for his magnanimous support during the course of the study. I give my sincere thanks to Dr. R. Manakadan (Deputy Director, BNHS), Dr. Alain J. Crivelli and Dr. Giorgos Catsadorakis (Chairman, Pelican Specialist Group (Old World)/Wetlands International– International Union for Conservation of Nature Species Survival Commission (WI-IUCN SSC) for their support. Special thanks to Dr. P.A. Azeez (Former Director, Sàlim Ali Centre for Ornithology and Natural History (SACON), and my friends H. Byju, Deepan Chakravarthy, Dr Soumya Dasgupta and Dr L. Prabha Devi for very useful comments on early version of the manuscript. Last but not least, I thank my field assistant, E. Munikrishna, for his assistance during field activities.
References
- Stephens, D.W., and Krebs, J.R., 1986. Foraging Theory. Princeton University Press, Princeton.
- Behavior, F., 1987. AC Kamil, JR Drebs, and HR Pulliam. New York., 69-140.
- Higginson, A.D., and Ruxton, G.D., 2015. Foraging mode switching: the importance of prey distribution and foraging currency. Anim. Behav., 105: 121-137.
- Bautista, L.M., Tinbergen, J., and Kacelnik, A., 2001. To walk or to fly? How birds choose among foraging modes. Proc. Natl. Acad. Sci., 98: 1089-1094.
- Pulliam, H.R., 1984. Living in groups: is there an optimal group size? Behav. Ecol., 122-147.
- Ruxton, G.D., Hall, S.J., and Gurney, W.S.C., 1995. Attraction toward feeding conspecifics when food patches are exhaustible. Am. Nat., 145: 653-660.
- Brown, C.R., and Brown, M.B., 1996. Coloniality in the cliff swallow: the effect of group size on social behavior. University of Chicago Press.
- Brown, C.R., 1988. Social foraging in cliff swallows: local enhancement, risk sensitivity, competition and the avoidance of predators. Anim. Behav., 36: 780-792.
- Clark, C.W., and Mangel, M., 1984. Foraging and flocking strategies: information in an uncertain environment. Am. Nat., 123: 626-641.
- Ekman, J., and Hake, M., 1988. Avian flocking reduces starvation risk: an experimental demonstration. Behav. Ecol. Sociobiol., 22: 91-94.
- Caraco, T., Barkan, C., Beacham, J.L., Brisbin, L., Lima, S., Mohan, A., and Withiam, M.L., 1989. Dominance and social foraging: a laboratory study. Anim. Behav., 38: 41-58.
- Kokshaysky, N.V., 1966. The role of behavior in formation of feeding habits in herons, Moscow, 231-245.
- Kushlan, J.A., 1972. Aerial feeding in the Snowy Egret. Wilson bull., 84: 199-200.
- Kushlan, J.A., 1973. Black-crowned Night Heron diving for prey. Fla. Field Nat., 1: 27-28.
- McFarland, D.J., 1977. Decision making in animals. Nature., 269: 15-21.
- Drent, R.H., and Daan, S., 1980. The prudent parent: energetic adjustments in avian breeding 1. Ardea., 55: 225-252.
- Pennycuick, C.J., 1975. Mechanics of flight. J. Avian Biol., 5: 1-73.
- Pennycuick, C., 1996. Wingbeat frequency of birds in steady cruising flight: new data and improved predictions. J. Exp. Biol., 199: 1613-1618.
- Kahl, M.P., 1971. Food and feeding behavior of Openbill Storks. J. Ornithol., 112: 21-35.
- Meyerriecks, A.J., 1962. Diversity typifies heron feeding. Nat. Hist., 71: 46-57.
- North, M.E.W., 1963. Breeding of the Black-headed Heron at Nairobi, Kenya. 1958-62. J. East Afr. Nat. Hist. Soc., 24: 33-63.
- Ali, S., and Ripley, S.D., 1968. Handbook of the birds of India and Pakistan: together with those of Nepal, Sikkim, Bhutan and Ceylon. Oxford University Press, 1.
- Blaker, D., 1969. Behaviour of the cattle egret Ardeola ibis. Ostrich., 40: 75-129.
- Carpenter, J.W., 1971. Notes on the biology and behavior of captive Boat-billed Herons, Cochlearius cochlearius. Southwest. Nat., 31-41.
- Siegfried, W.R., 1971. The food of the cattle egret. J. Appl. Ecol., 447-468.
- Dinsmore, J.J. 1973. Foraging success of cattle egrets, Bubulcus ibis. Am. Midl. Nat., 242-246.
- Ayres, T., and Gurney, J.H., 1878. Additional notes on the ornithology of Transvaal. Ibis., 20: 281-301.
- Loveridge, A., 1922. Notes on East African birds (chiefly nesting habits and stomach contents). Proc. Zool. Soc. Lond., 92: 837-862.
- Rand, A.L., 1954. Social feeding behavior of birds. Fieldiana Zool., 36: 1-71.
- Jackson, F.J., 1938. The Birds of Kenya Colony and the Uganda Protectorate: Completed and Ed. by WL Sclater. Gurney and Jackson.
- Delacour, J., 1946. Under-wing fishing of the Black Heron, Melanophoyx ardesiaca. Auk., 63: 441-442.
- Curry-Lindahl, K. 1961. Ecological Studies on Mammals, Birds, Reptiles and Amphibians in the Eastern Belgian Congo, Part II. Ornithology, 78: 105–106.
- Meyerriecks, A.J., 1960. Comparative breeding behavior of four species of North American herons. Auk, 77: 479.
- Du Plessis, S.S., 1963. The feeding behaviour of the Black Heron Melanophoyx ardesiaca. Ostrich., 34: 111-112.
- Kahl, M.P., 1964. Food ecology of the Wood Stork (Mycteria americana) in Florida. Ecol. Monogr., 34: 98-117.
- Del, H.J., Elliott, A., and Christie, D., 1992. Handbook of the birds of the world. Lynx ediciones, Barcelona. 1: 290-311.
- Nagulu, V., 1983. Feeding and breeding biology of Grey Pelican at Nelapattu bird sanctuary in Andhra Pradesh, India (Doctoral dissertation, Dissertation, Osmania University, Hyderabad, India).
- Ali, S., Ripley, S.D., and Dick, J.H., 1987. Compact handbook of the birds of India and Pakistan.
- Ali, S., 1960. Flamingo City” re-visited: Nesting of the Rosy Pelican (Pelecanus onocrotalus Linnaeus) in the Rann of Kutch. J. Bombay Nat. Hist. Soc., 57: 412-415.
- Brown, L.H., and Urban, E.K., 1969. The breeding biology of the great white pelican Pelecanus onocrotalus roseus at Lake Shala, Ethiopia. Ibis., 111: 199-237.
- Din, N.A.. and Eltringham, S.K., 1974. Ecological separation between White and Pink-backed Pelicans in the Rwenzori National Park, Uganda. Ibis., 116: 28-43.
- Brown, L.H., Urban, E.K., and Newman, K., 1982. The Birds of Africa. London: Academic Press. 1.
- Saino, N., Fasola, M., and Waiyakp, E., 1995. Do white pelicans Pelecanus onocrotalus benefit from foraging in flocks using synchronous feeding?. Ibis., 137: 227-230.
- Hatzilacou, D., 1996. Feeding ecology of the Great White Pelican (Pelecanus onocrotalus) nesting at lake Mikri Prespa (northwestern Greece). Col. Waterbirds., 190-206.
- Scott, D.A., 1989. A Directory of Asian Wetlands. IUCN.
- Islam, M.Z., and Rahmani, A.R., 2004. Important Bird Areas in India: Priority sites for Conservation. Bombay Nat. Hist. Soc., 157-158.
- Ali, S., and Ripley, S.D., 1978. Handbook of the birds of India and Pakistan. 2nd ed. Oxford University Press, Bombay. 29-30.
- Sen Nag, O., 2019. The Eight Extant Species of Pelicans.
- International, B., 2000. Threatened birds of the world. Lynx Editions, BirdLife International, Barcelona and Cambridge.
- Book, R.D., 2001. Threatened birds of Asia: the BirdLife International Red Data Book. UK. Cambridge, 1458-1477.
- Kannan, V., and Manakadan, R., 2005. Status and Distribution of the Spot-billed Pelican Pelecanus philippensis in southern India. Forktail., 21: 9-14.
- Archibald, G., 1992. A bird’s eye view of Cambodia. ICF Bugle., 18: 1-3.
- Scott, D.A., 1992. Survey of Cambodian wetlands. Unpublished.
- Carr, P., 1993. Bird observations from the southern reaches of the Tonle Sap Lake in central Cambodia from April 9th to 16th June 1993. Unpublished typescript.
- Mundkur, T., and Taylor, V., 1993. Asian Waterfowl Census 1993. Asian Wetland Bureau and International Waterfowl and Wetlands Research Bureau.
- Silvius, M.J., 1986. South-east Sumatra. Interwader Newsletter. 7.
- Verheugt, W.J., Skov, H., and Danielsen, F., 1992. Notes on the birds of the tidal lowlands and floodplains of South Sumatra province, Indonesia. Kukila., 6: 53-84.
- Boonsong, L., and Round, PD., 1991. A guide to the birds of Thailand. Saha. Karn. Bhaert.
- Weerd, M.V., and Der Ploeg, J.V., 2004. Surveys of wetlands and waterbirds in Cagayan valley, Luzon, Philippines. Forktail., 20: 33-39.
- Aarestrup, C., Andersen, P.A., Falck, J., Buhring, A., Meilstrup, H., and Tingvad, N., 1971. Systematic list of birds seen in India, Nepal, Pakistan, Afghanistan, Iran, USSR and Turkey, November 1970 to May 1971.
- Johnsgard, P., 1993. Cormorants, Darters, and Pelicans of the World. Washington: Smithsonian Institution Press.
- Grimmett, R., Inskipp, C., Inskipp, T., and Byers, C., 1999. Birds of India, Pakistan, Nepal, Bangladesh, Bhutan, Sri Lanka, and the Maldives. Princeton University Press, Princeton, NJ.
- Statters?eld, A., and Capper, D., 2000. Threatened Birds of the World. BirdLife International: Cambridge, UK.
- Hutchins, M., 2003. Grzimek's Animal Life Encyclopedia. Mammals 4.
- BirdLife International. 2004. Important bird areas in Asia: key sites for conservation. BirdLife International.
- BirdLife International. 2005. Species fact sheet: Pelecanus philippensis.
- UNEP., 2005. Proposed amendments to annexes to the Agreement’s text. Secretariat provided by the United Nations Environment Programme (UNEP).
- Crivelli, A.J., and Schreiber, R.W., 1984. Status of the Pelecanidae. Biol. Conserv., 30: 147-156.
- Collar, N.J., Crosby, M.J., and Stattersfield, A.J., 1994. Birds to watch 2: the world list of threatened birds. Cambridge, UK: BirdLife International. 4.
- Crivelli, A.J., and Anderson, D., 1996. WI/BirdLife/SSC Pelican Specialist Group. Species Survival Commission, IUCN.
- BirdLife International. (2003). Saving Asia's threatened birds: a guide for government and civil society. BirdLife International.
- Crosby, M.J., and Chan, S., 2006. Threatened waterbird species in eastern and southern Asia and actions needed for their conservation. Waterbirds around the world. Edinburgh, UK: The Stationery Office, 332-338.
- Kannan, V., and Pandiyan, J., 2013. A review on the spot-billed pelican Pelecanus philippensis literature. Front. Biol., 8: 333-352.
- BirdLife International., 2019. Species fact sheet: Pelecanus philippensis.
- Kushlan, J.A., 1978. Feeding ecology of wading birds. Natl. Audubon Soc., 249-297.
- Whitefield, A.K. and Blabber, S.J.M. 1979. Feeding ecology of piscivorous birds at Lake St Lucia, Part 3: Swimming birds. Ostrich., 50: 10-20.
- Kushlan, J.A., 1976. Feeding behavior of North American herons. Auk., 93: 86-94.
- Meyerriecks, A.J., 1959. Foot-stirring feeding behavior in herons. Wilson bull., 71: 153-158.
- Kushlan, J.A., 1973. Bill-vibrating: A prey-attracting behavior of the Snowy Egret, Leucophoyx thula. Am. Midl. Nat., 509-512.
- Mock, D.W., 1974. Aerial hunting by Little Blue Herons. Wilson bull., 86: 280-282.
- Kushlan, J.A., 1977. Population energetics of the American white ibis. Auk., 94: 114-122.
- Powell, G.V., 1987. Habitat use by wading birds in a subtropical estuary: implications of hydrography. Auk., 104: 740-749.
- Pianka, E.R., 1974. Niche overlap and diffuse competition. Proc. Natl. Acad. Sci., 71: 2141-2145.
- Murdy, H.W., 1966. When the prairies go dry. Naturalist., 17: 8-13.
- Krapu, G.L., 1974. Feeding ecology of pintail hens during reproduction. US Fish and Wildlife Service.
- Smith, K.D. 1970. The Waldrapp Geronticus eremita. Bull. Br. Ornithol. Club., 90: 18-24.
- Henny, C.J., Johnson, D.H., Bogan, M.A., Husar, S.L., and Sargeant, A.B., 1972. An analysis of the population dynamics of selected avian species: with special reference to changes during the modern pesticide era. Fish and Wildlife Service. 1.
- Brewster, W.G., Gates, J.M., and Flake, L.D., 1976. Breeding waterfowl populations and their distribution in South Dakota. J. Wildl. Manag.,40: 50-59.
- Stewart, R.E., and Kantrud, H.A., 1973. Ecological distribution of breeding waterfowl populations in North Dakota. J. Wildl. Manag., 37: 39-50.
- Pennak, R.W., 1953. Freshwater invertebrates of United States. Univ. Colorado.
- Stewart, R.E., and Kantrud, H.A., 1971. Classification of natural ponds and lakes in the glaciated prairie region. US Bureau of Sport Fisheries and Wildlife. 92: 57.
- Andersson, G., 1981. Influence of fish on waterfowl in lakes. Anser., 20: 21-34.
- Findholt, S.L., and Anderson, S.H., 1995. Foraging areas and feeding habitat selection of American White Pelicans (Pelecanus erythrorhynchos) nesting at Pathfinder Reservoir, Wyoming. Col. Waterbirds., 47-57.
- Anderson, J.G., 1991. Foraging behavior of the American white pelican (Pelecanus erythrorhyncos) in western Nevada. Col. Waterbirds., 166-172.
- Kadlec, J.A., 1962. Effects of a drawdown on a waterfowl impoundment. Ecology, 43: 267-281.
- Marshall, R.V.A., 1961. Herons fishing from the air. Brit. Birds, 54: 202.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref