Review Article - Journal of Orthopedic Surgery and Rehabilitation (2017) Volume 1, Issue 1
Sacral insufficiency fractures
- *Corresponding Author:
- Mario Alberto Cahueque Lemus
Orthopedic Surgeon Centro Medico, Guatemala Republic of Guatemala
Accepted date: December 22, 2016
Citation: Cobar-Bustamante A, Lemus MAC, Bregni-Duraes M, et al. Sacral insufficiency fractures. J ortho Rehab Surg. 2016;1(1):1-6.
Abstract
Sacral insufficiency fractures (SIF) is a common cause of debilitating back pain in the elderly. First described by Lourie in 1982. Since then, there has been much more cases reported due to the awareness of this entity, however, there is often a delay in diagnosis because clinical symptoms are commonly vague and nonspecific, these can mimic a variety of pathologies. These fractures can cause considerable pain, loss of independence, and economic burden to both the patient and the health care system. Bone scintigraphy and MR are the most sensitive studies to detect SIFs, though findings have been described in a variety of radiologic imaging. The standard care for the treatment of SIFs has been conservative management, with bed rest, analgesics, and rehabilitation. Sacroplasty has been proposed as an alternative to conservative treatment. Studies suggest that it is a safe and effective procedure, providing early symptomatic relief. The role of operative fixation in insufficiency fractures is also discussed.
Keywords
Sacral insufficiency, Fractures, Elderly, Sacroplasty.
Introduction
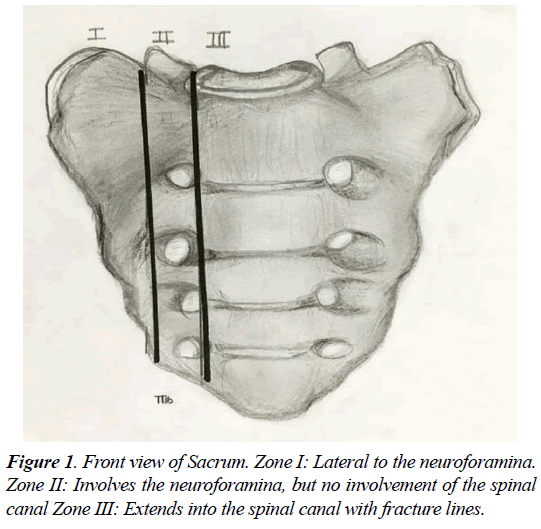
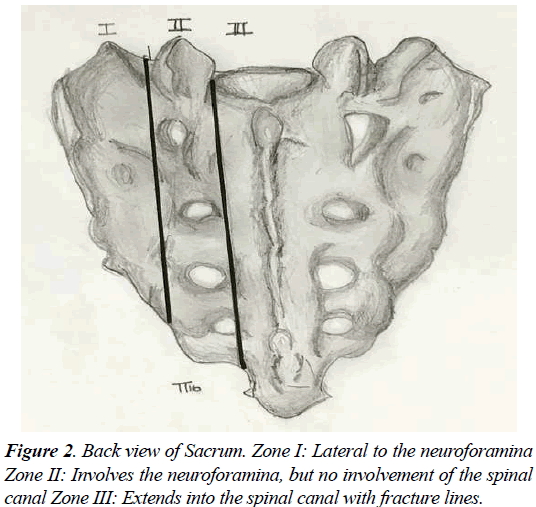
Sacral insufficiency fractures (SIF) is a common cause of debilitating back pain in the elderly. First described by Lourie in 1982 [1]. Stress fractures occur in the presence of repetitive forces over time, that result in a failure of the integrity of the skeletal structures to withstand the trauma [2-4]. Two types of stress fractures can be identified, insufficiency and fatigue fractures. Insufficiency fractures occur in the setting of physiological loads upon weakened bone (suboptimal bone mass, quality, or composition) [4]. Fatigue fractures are due to abnormal force and load applied to optimum bone quality and structure, in a repetitive manner that leads to an injury. Sacral insufficiency fractures are commonly misdiagnosed, for an adequate level of suspicion and careful diagnostic imaging workup is required to detect them. The sacrum, located in the caudal portion of the spine, is composed of a body and two sacral ala. The Sacral fractures can be described by their anatomic location in three zones (Figures 1 and 2). Zone 1 corresponds to the ala, and is the location of most insufficiency fractures. These fractures rarely involve the foramina, as in the setting of zone 2 fractures. Zone 3 fractures are in the central sacral canal and are frequently accompanied by neurological deficit, and saddle anaesthesia [2-6].
Insufficiency fractures of the sacrum are rarely diagnosed. These fractures occur majorly in a vertical pattern, parallel to the sacroiliac joint and in line with axial load vectors. As the load continues, the sacral body may give way to a transverse fracture, leading to further instability [5,6].
Etiology
Lourie first described sacral insufficiency fractures in 1982 [1]. Insufficiency fractures are a subtype of stress fracture that results from normal stress applied to abnormal bone that has lost its elastic resistance. Bone insufficiency is often the result of osteoporosis or other metabolic bone disease, though osseous metastatic disease and marrow replacement processes can also cause insufficiency fractures [4,6,7]. SIFs (sacral insufficiency fractures) most commonly involve the sacral ala, lateral to the neural foramina and medial to the sacroiliac joints. Fractures may be unilateral or bilateral and are reported with relatively equal frequency in the literature [2-4,6]. There may also be a horizontal component to the fracture through the sacral bodies. This unique fracture pattern may be related to axial loading and weight-bearing transmitted through the spine, resulting in sacral alar strain; this horizontal fracture, seen on bone scintigraphy of magnetic resonance imaging (MRI), is called the H or “Honda sign) [8]. Additionally, osteoporosis causes asymmetric loss of bony trabecular in the sacral ala compared with the vertebral bodies, placing the lateral aspect of the sacrum at increased risk of insufficiency [7,9-11]. The force required to produce a sacral insufficiency fracture is unknown. Typical forces across the hip joint during walking are 3 times body weight, whereas a simple stumble may produce forces up to 8 times body weight. Waites et al. have hypothesized that the vertical load required to produce the pattern of fractures seen clinically in patients with sacral insufficiency fractures was consistent with normal physiologic forces12.The recovery may be prolonged, and some patients develop chronic pain and non-union requiring surgery. The 1-year mortality rate is reported as 14.3%. On discharge, 50% had not recovered their pre- injury level of self-sufficiency and 25% required institutionalization. Sacral insufficiency fractures also have been observed after extended fusions of the lumbar spine [7,11,12].
Epidemiology
SIFs commonly affect elderly women with osteoporosis, though other reported risk factors include pelvic radiation, steroidinduced osteopenia, rheumatoid arthritis, multiple myeloma, Paget disease, renal osteodystrophy, hyperparathyroidism, as well as long fusions of the spine. Of these, osteoporosis is the most prevalent, and almost all patients with SIFs will demonstrate severe osteopenia on dual X-ray absorptiometry, even if other risk factors are present [3,5,10,12].
Prior pelvic radiation is another well-established risk factor for the development of SIFs, with a reported prevalence of 21% to 34%. The incidence may be as high as 89%, as suggested by a prospective study of patients undergoing pelvic radiation for cervical cancer [9,13].
Almost all patients with SIFs are older than 55 years of age, with a mean age between 70-75 years in most studies. The true incidence of SIFs is unknown but has been reported to be between 1% and 5% in at-risk patient populations. Antecedent trauma is not identified in two-thirds of patients and, when present, is usually minor [4,10,12,14]. This type of fracture is becoming more common with the increase in life expectancy. Close to 75% of patients who present to the hospital with sacral fractures are neurologically intact; thus, the diagnosis is often missed on the initial visit, and patients do not receive optimal treatment [15,16]. These patients may go on to develop neurological deficits due to inadequate treatment.
Patients with SIFs most commonly present with diffuse low back pain, which may radiate to the buttock, hip, or groin. Patients may have some tenderness to palpation in the lower back and sacral region, though this is not a consistent finding, they may also have coexisting fragility fractures such as vertebral compression fractures and pubic rami fractures. Neurologic symptoms related to SIFs are unusual, though may be seen in 5% to 6% of patients, most commonly manifesting as sacral radiculopathy. However, a case of cauda equina syndrome related to SIFs has been reported [3-7,10,13,15,16].
SIFs can occasionally be confused with metastatic disease, both clinically and on imaging studies, resulting in unnecessary work-up and biopsy. This is frequently a confounding factor in elderly patient populations, many of whom have known primary malignancy or are being evaluated for an occult tumor. In fact, approximately 45% of patients with SIFs have a history of malignancy [15-17].
Imaging
Initial imaging is often not targeted to the sacrum, but commonly to the lumbar spine. The mean delay of symptom onset to sacral imaging has been reported from 40-55 days [16,17].
Plain X-Ray
Findings range from sclerosis to cortical disruption. Presence of sclerotic fracture lines can range from 1 to 13 months. In some cases, the periosteal reaction can lead to unnecessary work-up and biopsy, simulating malignancy. X-ray films are insensitive for the detection of SIFs. Studies suggest that only 20% to 38% of the SIFs and pelvic ring fractures are identified on plain films [14-18].
Nuclear Medicine
Bone scintigraphy with technetium TC99m- labeled MDP is one of the most sensitive examinations for the detection of SIFs. The “Honda” sign or H-pattern considered diagnostic of SIFs in the correct clinical setting [8,18-20]. However, it is only present in 20% to 40% of patients. Variations include unilateral sacral uptake, unilaterally with horizontal strut, bilaterally without horizontal strut, and multiple foci of activity. Follow-up MDP is variable, ranging from no activity to abnormal or augmented activity in a 10-33 months’ interval. Sensitivity has been reported in 96% with a positive predictive value of 92% [20].
CT
Fracture lines with or without callus can be seen in 75% of the patients. Most fractures are sagittal oriented and are clear, however, the full extent of the sacral fracture may not be evident if there is an isolated or significant horizontal component, for this reason coronal images are helpful [21]. CT has a reported sensitivity between 60% and 75%. Bone detail exposed on CT can help determine the involvement of neural foramina, creating potential pathway for cement if a Sacroplasty is being considered. It can also be helpful to differentiate from metastatic disease [20,21].
MRI
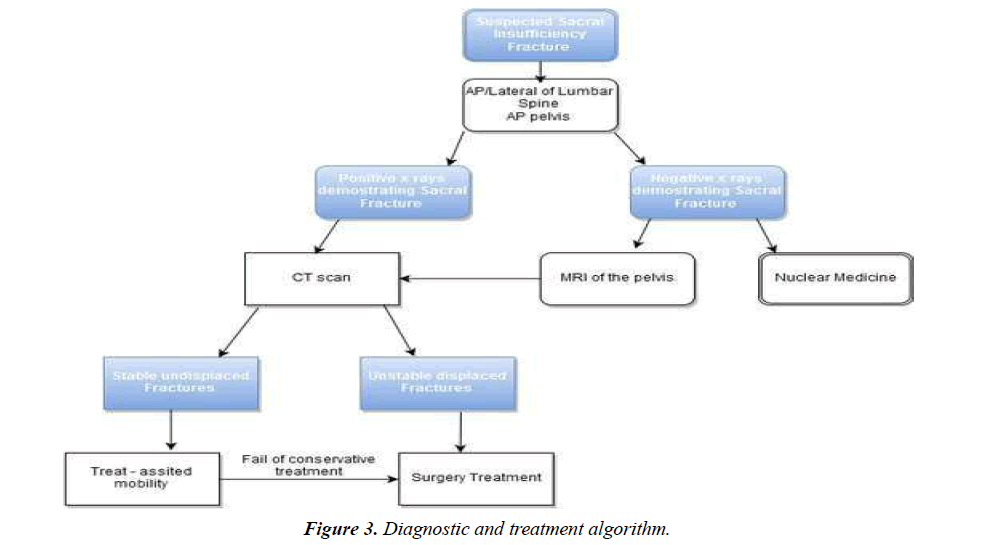
MR can detect early chances of sacral insufficiency and, like bone scintigraphy, has a reported sensitivity at or near 100%. Marrow edema as early as 18 days after symptoms develop. Mostly surrounding fracture line, which be increased intensity on T2-weighted and inversion-recovery images and low signal intensity on T1-weighted images. Coronal oblique images are best to detect vertically oriented fractures. MRI can differentiate malignancy from fracture edema in SIFs with fat-saturation and post-gadolinium imaging. Usually there can be confusion generated by MRI alone, which is why there should be a CT to correlate the findings. Spin-Echo sequence MRI is very sensitive for pathologic fractures [6,10,16,18,22] (Figure 3).
Treatment
Conservative therapy
Conservative treatment is indicated mainly stable undisplaced fractures. Conservative treatment has been the standard of care in SIFs, though treatment options are variable. Some authors recommend strict bed rest and pain control. Others recommend moderation of activity, crutches, or a walker in addition to analgesics [12,23]. Some reports promote early physical rehabilitation. Even though most patients show improvement following conservative management, the time course can be prolonged and quite variable, as well as it can be non-favourable. Most patients report symptoms to resolve from 12 to 15 months [23].
Prolonged bed rest can be related to significant morbidity, especially in elderly patients. The primary concern is Venous Thrombo-Emolism (VTE), which has been reported between 29% and 61% of patients and Pulmonary Embolism (PE) in 2% to 12%. Other complications include loss of muscle mass, cardiac dysfunction, pneumonia, decubitus ulceration and bone demineralization. It has been reported that almost half of the patients with SIFs will not return to prior functional level, and a mortality of 14.3% [19,24].
There are 3 major components that should be addressed by orthopedic surgeons:
1. Providing an adequate calcium and vitamin D environment to facilitate well-mineralized bone and improve bone quality.
2. Preventing excessive bone resorption (Biphosphonates).
3. Providing an anabolic stimulus to enhance bone formation. (Teripartide) [25,26].
Sacroplasty
Sacroplasty has emerged as a minimally invasive alternative to conservative therapy for SIFs. Offering pain relief by limiting micromotion between fragments and thermochemical neurotoxic effect on nerve endings like vertebroplasty in the thoracolumbar spine, with the use of PMMA cement into the fractured sacrum under imaging guidance. The exact mechanism by which it relieves pain is not completely understood [27,28].
The goal of Sacroplasty is to provide early symptomatic relief, allowing more rapid mobilization, limiting the need for significant narcotic analgesics, and lessen the risks associated with prolonged bed rest. Sacroplasty can safely and effectively provide early symptomatic relief, though these findings have not been verified in controlled prospective randomized trials. It has not yet achieved widespread acceptance, unlike vertebroplasty, probably due to the lack of validating controlled studies or because of unique technical considerations related to sacral anatomy [28-30].
The technical aspects of Sacroplasty vary significantly between operators, including the technique of imaging guidance, as well as the needle approach and the long-axis approach. A midline approach has also been used by some physicians to treat the horizontal or sacral body (zone 3) component of the fracture. Although the exact technique of these different approaches is beyond the scope of this article, we have described the advantages and disadvantages of each technique in Table 1.
| Technique | Advantages | Disadvantages |
|---|---|---|
| Posterior | Familiarity | Invasion of anterior sacral cortex |
| Well described fluoroscopic landmarks | Increased risk of cement extravasation | |
| Long Axis | Removes risk of invasion of ventral cortex | Technique is more demanding |
| Cement can be extruded from the procedure needle more evenly | ||
| Midline | Better in horizontal components of SIFs | Needs a preoperative MR to verify that the needle is below the caudal extent of the thecal sac |
Table 1: Advantage vs. disadvantage for sacroplasty.
Safety and efficacy
Sacroplasty has provided early subjective symptomatic relief in patients treated for SIFs. The prospective study of 52 patients treated with Sacroplasty found a 50% reduction in the VAS at 2 days, 80% at 2 weeks, and 90% at 1 year [31]. Additionally, the authors found that reduction in the VAS paralleled the reduction in the use of narcotic medication. In addition, patients reported improved ability to perform activities of daily living. Although these results are promising, they have not been prospectively compared with those of a control group of patients treated with conservative therapy [28,31,32].
Sacroplasty has also been used to treat painful metastatic lesions in the sacrum. Sacral cement injection for hepatocellular carcinoma, haemangioma, lung cancer, lymphoma, renal cell carcinoma and myeloma [27,32,33].
Complications of sacroplasty
Unexpected extrusion of PMMA cement outside of the fractured sacrum, with untoward neurologic sequelae being the highest concern (7.4%) [33-35].
• Cement extrusion into the para-spinal soft tissues and sacroiliac joints.
• Extrusion of cement into the sacral spinal canal with associated neurologic compromise.
• Infection.
• Venous emboli.
In case of cement leakage, surgical decompression has been reported [32]. Although documented complications of sacroplasty are often cited as being clinically insignificant, cases of sacral and lumbar radiculopathy due to cement extravasation have been reported, one of which resolved with nerve root injection of a steroid and local anesthetic combination. Risk factors identified are: low viscosity of injected PMMA, overfilling, inadequate needle placement and lack of imaging guidance.
Lumbo-pelvic fixation
Lumbo-pelvic fixation has been reported. Lumbar pedicle screws and double iliac screws have been used with successful fixation. This technique is preferred when SIFs are adjacent to long lumbar instrumentations with an incidence of 3.1%. Recommendations include fusion with autologous bone grafting versus augmentation with bone cement along with decompression. Indications are reserved for fractures with severe displacement, sagittal imbalance, neurologic symptoms or painful non-union. Fixation with short long transiliac screws have also been described [36,37].
Other Treatment Options
Sacroplasty with balloon and sacral kyphoplasty have been reported. Sacral kyphoplasty is technically like sacroplasty, and several balloon systems are commercially available [38-41]. One suggested advantage of sacral kyphoplasty is the creation of a compacted bony layer outside the balloon, which could theoretically lower the likelihood of cement extravasation. Fluoroscopic and computer assisted percutaneous placement of transiliosacral screws has also been reported [42-45].
A comprehensive strategy for the improved treatment of osteoporotic fractures must address both biological and mechanical issues and includes 4 specific approaches [46,47].
1. Removal of inhibitors to bone healing.
2. Introduction of bone healing stimulants.
3. Application of bone augmentation or substitutes.
4. Modification of fracture fixation constructs.
Known inhibitors of bone healing (such as smoking, alcohol, anti-inflammatory medications, and steroids) [48] should be removed, together with optimal control of medical issues (including malnutrition, diabetes, infection, and thyroid disease). Stimulation of bone healing can be achieved through surgical (bone marrow aspirates, platelet gels, and bone morphogenetic proteins), medical (Vitamin-D, Calcium, Bisphosphonates, and PTH) [49], or physical means (Ultrasound, Direct electrical stimulation, Pulsed electromagnetic fields, and Extracorporeal shock) [50].
Conclusions
Pelvic insufficiency fractures are increasing in incidence and can cause considerable disability in the elderly patients. Many studies indicate prolonged periods of immobility, the potential for complications, and loss of independence. The accurate workup and diagnosis of sacral insufficiency fractures is important to prevent the need for costly, painful, and otherwise invasive methods of diagnosis. In patients with underlying bone disease, sacral insufficiency fractures must always be a diagnostic consideration, and the importance in differentiating these fractures with fatigue fractures, results in its optimum management. Clinical examination is important, and nonspecific findings can be evident, leading to further studies. Plain X-ray film and Computed Tomography Scanning are useful, but Magnetic Resonance Imaging is the method of choice in the suspicion and detection of these fractures.
Patients with insufficiency fractures must be treated medically, with calcium and vitamin D, bisphosphonates and calcitonin, as osteoporosis is the main risk factor for fracture incidence. Moderate weight bearing and exercise are an important part of physical rehabilitation. Operative intervention is rarely indicated. Patients with insufficiency fractures generally have a satisfactory functional outcome, given the treatment of the underlying diseases, and adequate symptom management.
Conflict of Interest
None.
References
- Lourie H. Spontaneous osteoporotic fracture of the sacrum: An unrecognized syndrome in the elderly. JAMA 1982;248:715–17.
- Gotis-Graham I, McGuigan L, Diamond T, et al. Sacral insufficiency fractures in the elderly. J Bone Joint Surg BR 1994;76:882–86.
- Peh WC, Khong PL, Ho WY,et al. Sacral insufficiency fractures: spectrum of radiological features. Clin Imaging 1995;19(2):92–101.
- Finiels H, Finiels PJ, Jacquot JM, et al. Fractures of the sacrum caused by bone insufficiency: Meta-analysis of 508 cases. Presse Med. 1997;26(33):1568-73.
- Grangier C, Garcia J, Howarth NR, et al. Role of MRI in the diagnosis of insufficiency fractures of the sacrum and acetabular roof. Skeletal Radiol. 1997;26(9):517–24.
- DeSmetAA, Neff JR. Pubic and sacral insufficiency fractures: clinical course and radiologic findings. AJR Am J Roentgenol. 1985;145(3):601-6.
- Blake SP, Connors AM. Sacral insufficiency fracture. Br J Radiol. 2004;77(922):891-96.
- Fujii M, Abe K, Hayashi K, et al. Honda sign and variants in patients suspected of having a sacral insufficiency fracture. ClinNucl Med.2005;30(3):165-69.
- Lundin B, Björkholm E, Lundell M,et al. Insufficiency fractures of the sacrum after radiotherapy forgynaecological malignancy. ActaOncol. 1990;29(2):211–15.
- Bustamante-VidalesJC, Kleriga-Grossgere E, Zambito-BrondoGF, et al. Sacral insufficiency, unexpected clinical entity as a cause of low back pain. Report of two cases. Cir Cir. 2012;80(6):556-61.
- Waites MD, Mears SC, Mathis JM,et al. The strength of the osteoporotic Spine. Phila Pa. 2007;32(23):E652-55.
- Tsiridis E1, Upadhyay N, Giannoudis PV. Sacral insufficiency fractures: current concepts of management. Osteoporos Int. 2006;17(12):1716-25.
- Bydon M, De La Garza-Ramos R, Macki M,et al. Incidence of sacral fractures and in-hospital postoperative complications in USA. Spine. 2014;39(18):E1103-9.
- Jeray K,Swiontkowski M. Osteoporosis and fragility fractures. J Bone Joint Surg Am. 2015;97(19):1553-4.
- Lee YJ, Bong HJ, Kim JT,et al. Sacral insufficicency fracture, usually overlooked cause of lumbosacral pain. J Korean Neurosurg Soc. 2008;44(3):166-69.
- Dasgupta B, Shah N, Brown H, et al. Sacral insufficiency fractures: An unsuspected cause of low back pain. Br J Rheumatol. 1998;37(7):789-93.
- Finiels PJ, Finiels H, Strubel D, et al. Spontaneous osteoporotic fractures of the sacrum causing neurological damage. J Neurosurg. 2002;97:380-85.
- Ferenc M, Puhl M, Varga PP. Sacral insufficiency fractures. IdeggyogySz.2013;66(7-8):235-41.
- Peh WC1, Khong PL, Yin Y,et al. Imaging of pelvic insufficiency fractures. Radiographics. 1996;16(2):335-48.
- CabarrusMCl, Ambekar A, Lu Y, et al. MRI and CT of insufficiency fractures of the pelvis and the proximal femur. AJR Am J Roentgenol. 2008;191(4):995-1001.
- Featherstone T. Magnetic resonance imaging in the diagnosis of sacral stress fracture. Br J Sports Med 1999;33(4):276-77.?
- Brahme SK, Cervilla V, Vint V, et al. Magnetic resonance appearance of sacral insuffiency fractures. Skeletal Radiol. 1990;19(7):489-93.
- Newhouse KE, El-Khoury GY, Buckwalter JA. Occult sacral fractures in osteopenic patients. J Bone Joint Surg Am 1992;74:1472-77.?
- Babayev M, Lachmann E, Nagler W. The controversy surrounding sacral in-sufficiency fractures: to ambulate or not to ambulate. Am J Phys Med Rehab. 2000;79:404-09.?
- Taillandier J, Langue F, Alemanni M, et al. Mortality and functional outcomes of pelvic insufficiency fractures in older patients. Joint Bone Spine.2003;70(4):287-89.
- Lehman RA Jr, Kang DG, Wagner SC. Management of osteoporosis in spine surgery. J Am AcadOrthop Surg. 2015;23(4):253-63.
- Longhino V, Bonora C, Sansone V. The mangement of sacral stress fractures: Current concepts. Clin Cases Miner Bone Metab. 2011;8(3):19-23.
- Bayley E, Srinivas S, Boszczyk BM. Clinical outcomes of sacroplasty in sacral insufficiency fractures: A review of the literature. Eur Spine J. 2009;18(9):1266-71.
- Andresen R, Lüdtke CW, Radmer S,et al. Radiofrequency sacroplasty (RFS) for the treatment of osteoporotic insufficiency fractures. Eur Spine J. 2015;24(4):759-63.
- Moussazadeh N, Laufer I, Werner T,et al. Sacroplasty for cancer-associated insufficiency fractures. Neurosurgery. 2015;76(4):446-50.
- Garant M. Sacroplasty: A new treatment for sacral insufficiency fracture. J VascIntervRadiol. 2002;13 (12):1265-67.
- Frey ME, Depalma MJ, Cifu DX,et al. Percutaneous sacroplasty for osteoporotic sacral insufficiency fractures: A prospective, multicenter, observational pilot study. Spine J. 2008;8(2):367-73.
- Gupta AC, Chandra RV, Yoo AJ,et al. Safety and effectiveness of sacroplasty: A large single-center experience. AJNR Am J Neuroradiol. 2014;35(11):2202-6.
- Dehdashti AR, Martin JB, Jean B,et al. PMMA cementoplasty in symptomatic metastatic lesions of the S1 vertebral body. CardiovascInterventRadiol. 2000;23(3):235-37.
- Barber SM, Livingston AD, Cech DA. Sacral radiculopathy due to cement leakage from percutaneous sacroplasty, successfully treated with surgical decompression. J Neurosurg Spine. 2013;18(5):524-28.
- Klineberg E, McHenry T, Bellabarba C,et al. Sacral insufficiency fractures caudal to instrumented posterior lumbosacral arthrodesis. Spine. 2008;33(16):1806-11.
- Mears SC, Sutter EG, Wall SJ,Et al. Biomechanical comparison of three methods of sacral fracture fixation in osteoporotic bone. Spine. 2010;35(10):E392-95.
- Deen HG, Nottmeier EW. Balloon kyphoplasty for treatment of sacral insufficiency fractures. Report of three cases. Neurosurg Focus. 2005;18(3):e7.
- Lüdtke CW, Kamusella P, Andresen R. CT-guided transiliac balloon kyphoplasty to supply an isolated Denis II insufficiency fracture of the sacrum. Rofo. 2013;185(7):664-65.
- Betts A. Sacral vertebral augmentation: Confirmation of fluoroscopic landmarks by open dissection. Pain Physician. 2008;11(1):57-65.
- Briem D, Grossterlinden L, Begemann PG. et al. CT-guided balloon-assisted sacroplasty. Preliminary results of a feasibility study. Unfallchirurg. 2008;111(6):381-86.
- Tjardes T, Paffrath T, Baethis H. et al. Computer assisted percutaneous placement of augmented iliosacral screws: a reasonable alternative to sacroplasty. Spine. 2008;33(13):1497-1500.
- Sciubba DM, Wolinsky JP, Than KD,et al. CT fluoroscopically guided percutaneous placement of transiliosacral rod for sacral insufficiency fracture: case report and technique. AJNR Am J Neuroradiol. 2007;28 (8):1451-54.
- Vanderschot P, Kuppers M, Sermon A,et al. Trans-iliac-sacral-iliac-bar procedure to treat insufficiency fractures of the sacrum. Indian J Orthop. 2009;43(3):245-52.
- Vanderschot P, Meuleman C, Lefevre A, et al. Trans iliac-sacral-iliac bar stabilisation to treat bilateral lesions of the sacro-iliac joint or sacrum: anatomical considerations and clinical experience. Injury. 2001;32(7):587-92.
- Dempster DW, Cosman F, Kurland ES,et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: A paired biopsy study. J Bone Miner Res. 2001;16(10):1846-53.
- McClung MR, Geusens P, Miller PD,et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip intervention program study group. N Engl J Med. 2001;344(5):333-40.
- Lauing KL, Roper PM, Nauer RK, et al. Acute alcohol exposure impairs fracture healing and deregulates β-catenin signaling in the fracture callus. Alcohol ClinExp Res. 2012;36(12):2095-103.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-81.
- Rutten S, Van Den Bekerom MP, Sierevelt IN,et al. Enhancement of bone-healing by low-intensity pulsed ultrasound: A systematic review. JBJS Rev. 2016;4(3).