Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2024) Volume 14, Issue 103
RP-HPLC Method for simultaneous Estimation of Metformin and lingaliptin in Pharmaceutical formulation
Erri Prashanth Reddy1*, Dr. G. Nagamallika21Deaprtment of Pharmaceutical analysis, Osmania University, Hyderabad Telangana, India
2Department of Pharmaceutical Chemistry, Omega college of pharmacy, Ghatkasar, India
- *Corresponding Author:
- Erri Prashanth Reddy
Deaprtment of Pharmaceutical analysis
Osmania University
Hyderabad Telangana, India
E-mail: prashanthreddypr072@gmail.com
Received: 25-Dec-2023, Manuscript No. AABPS-23-123588; Editor assigned: 28-Dec-2023, PreQC No. AABPS-23-123588(PQ); Reviewed: 11-Jan-2024, QC No. AABPS-23-123588; Revised: 16-Jan-2024, Manuscript No. AABPS-23-123588 (R); Published: 28-Jan-2024, DOI:10.35841/aabps-14.103.214
Citation: Reddy PE. RP-HPLC Method for simultaneous Estimation of Metformin and lingaliptin in Pharmaceutical formulation. Asian J Biomed Pharm Sci. 2024;14(103):214
Abstract
A simple, Accurate, precise method was developed for the simultaneous estimation of the Linagliptin and Metformin in Tablet dosage form. Chromatogram was run through Ascentis C18 150 x 4.6 mm, 5?m. Mobile phase containing Buffer: Acetonitrile taken in the ratio 65:35 was pumped through column at a flow rate of 0.9 ml/min. Buffer used in this method was 0.1% OPA (2.2ph) buffer. Temperature was maintained at 30°C. Optimized wavelength selected was 216.0nm Retention time of Linagliptin and Metformin were found to be 2.965 min and 2.247 min. %RSD of the Linagliptin and Metformin were and found to be 0.9 and 0.7 respectively. %Recovery was obtained as 100.04% and 99.66% for Linagliptin and Metformin respectively. LOD, LOQ values obtained from regression equations of Linagliptin and Metformin were 0.03, 0.05 and 0.002, 0.005 respectively. Regression equation of Linagliptin is y = 572521x + 1811.1.and y = 132021x + 122105 of Metformin. Retention times were decreased and that run time was decreased, so the method developed was simple and economical that can be adopted in regular Quality control test in Industries.
Key words
Metformin, Linagliptin, RP-HPLC
Introduction
The quality of a drug plays an important role in ensuring the safety and efficacy of the drugs. Quality assurance and control of pharmaceutical and chemical formulations is essential for ensuring the availability of safe and effective drug formulations to consumers. Hence Analysis of pure drug substances and their pharmaceutical dosage forms occupies a pivotal role in assessing the suitability to use in patients. The quality of the analytical data depends on the quality of the methods employed in generation of the data [1] . Hence, development of rugged and robust analytical methods is very important for statutory certification of drugs and their formulations with the regulatory authorities.
The quality and safety of a drug is generally assured by monitoring and controlling the assay and impurities effectively. While assay determines the potency of the drug and impurities will determine the safety aspect of the drug. Assay of pharmaceutical products plays an important role in efficacy of the drug in patients.
The wide variety of challenges is encountered while developing the methods for different drugs depending on its nature and properties. This along with the importance of achieving the selectivity, speed, cost, simplicity, sensitivity, reproducibility and accuracy of results gives an opportunity for researchers to come out with solution to address the challenges in getting the new methods of analysis to be adopted by the pharmaceutical industry and chemical laboratories. Different physico-chemical methods are used to study the physical phenomenon that occurs as a result of chemical reactions. Among the physico-chemical methods, the most important are optical (refractometry, polarimetry, emission and fluorescence methods of analysis), photometry (photocolorimetry and spectrophotometry covering UV- Visible, IR Spectroscopy and nepheloturbidimetry) and chromatographic (column, paper, thin layer, gas liquid and high performance liquid chromatography) methods. Methods such as nuclear magnetic resonance (NMR) and para magnetic resonance (PMR) are becoming more and more popular. The combination of mass spectroscopy (MS) with gas chromatography is one of the most powerful tools available. The chemical methods include the gravimetric and volumetric procedures which are based on complex formation; acid-base, precipitation and redox reactions. Titrations in non-aqueous media and complexometry have also been used in pharmaceutical analysis. The number of new drugs is constantly growing. This requires new methods for controlling their quality. Modern pharmaceutical analysis must need the following requirements.
1. The analysis should take a minimal
2. The accuracy of the analysis should meet the demands of
3. The analysis should be
4. The selected method should be precise and
Chromatography
Chromatography (Chroma means ‘color’ and graphein means to ‘write’) is the collective term for a set of laboratory techniques for the separation of mixtures. It involves passing a mixture dissolved in a "mobile phase" through a stationary phase [2-5]. Which separates the analyte to be measured from other molecules in the mixture based on differential partitioning between the mobile and stationary phases. Differences in compounds partition coefficient results in differential retention on the stationary phase and thus changing the separation.
Different types of chromatographic techniques were summarized in (Table 1)
| Sl. No | Basic principle involved | Type of Chromatography |
|---|---|---|
| 1 | Techniques by chromatographic bed shape |
Column chromatography |
| Paper chromatography | ||
| Thin layer chromatography | ||
| 2 | Techniques by physical state of mobile phase | Gas chromatography |
| Liquid chromatography | ||
| 3 | Affinity chromatography | Supercritical fluid chromatography |
| 4 | Techniques by separation mechanism | Ion exchange chromatography |
| Size exclusion chromatography | ||
| 5 | Special techniques | Reversed phase chromatography |
| Simulatedmoving-bed chromatography | ||
| Pyrolysis gas chromatography | ||
| Fast protein liquid chromatography | ||
| Counter current chromatography | ||
| Chiral chromatography |
Table 1: Different types of chromatographic techniques
Chromatography may be preparative or analytical. The purpose of preparative chromatography is to separate the components of a mixture for further use (and is thus a form of purification). Analytical chromatography is done normally with smaller amounts of material and is for measuring the relative proportion of analytes in a mixture.
High performance liquid chromatography (HPLC)
Liquid chromatography is an analytical chromatographic technique that is useful for separating ions or molecules that are dissolved in a solvent. If the sample solution is in contact with a second solid or liquid phase to differing degrees due to differences in adsorption, ion exchange, partitioning or size. These differences will allow the mixture components to be separated from each other by using these differences to determine the time of the solutes through a column. During 1970’s, most chemical separations were carried out using a variety of techniques including open-column chromatography, paper chromatography and thin layer chromatography (TLC). However, these chromatographic techniques were inadequate for quantification of compounds and resolution between similar compounds. During this time pressure liquid chromatography began to be used to decreased flow through time, thus reducing separation time of compounds being isolated by column chromatography. However, flow rates were inconsistent, and the question of whether it was better to have constant flow rate or constant pressure debated. High pressure liquid chromatography quickly improved with the development of column packing materials. Additional convenience of on- line detectors became rapidly a powerful separation technique and is today called as High Performance Liquid Chromatography (HPLC).
Classification of HPLC
Based on modes of chromatography
• Normal phase chromatography
• Reverse phase chromatography
Based on principle of separation
• Adsorption chromatography
• Partition chromatography
• Ion exchange chromatography
• Size exclusion chromatography
• Affinity chromatography
• Chiral phase chromatography
Based on elution technique
• Isocratic separation
• Gradient separation
Based on the scale of operation
• Analytical HPLC
• Preparative HPLC
Normal phase high performance liquid chromatography (NP-HPLC)
NP-HPLC explores the differences in the strength of the polar interactions of the analytes in the mixture with the stationary phase. The stronger the analyte-stationary phase interaction, the longer the analyte retention. Analyte molecules compete with the mobile phase molecules for the adsorption sites on the surface of the stationary phase. The stronger the mobile phase interactions with the stationary phase, the lower the difference between the stationary phase interactions and the analyte interactions, and thus the lower the analyte retention. Mobile phases in NP-HPLC are based on non-polar solvents (such as hexane, heptanes, etc.) with the small addition of polar modifier (i.e., methanol, ethanol). Packing materials traditionally used in NP-HPLC are usually porous oxides such as silica (SiO2) or alumina (Al2O3). Surface of these stationary phases is covered with the dense population of OH groups, which makes these surfaces highly polar. Chemically modified stationary phases can also be used in NP-HPLC. Silica modified with trimethoxy glycidoxypropyl silanes (common name: diol-phase) is typical packing material with decreased surface polarity. Since NP-HPLC uses mainly non-polar solvents, it is the method of choice for highly hydrophobic compounds (which may show very stronger interaction with non polar mobile phases), which are insoluble in polar or aqueous solvents [6-9].
Reversed phase high performance liquid chromatography (RP-HPLC)
As opposed to NP-HPLC, RP-HPLC employs mainly dispersive forces (hydrophobic or vanderwal’s interactions). The polarities of mobile and stationary phases are reversed, such that the surface of the stationary phase in RP-HPLC is hydrophobic and mobile phase is polar , where mainly water-based solutions are employed. RP-HPLC is by far the most popular mode of chromatography. Almost 90 % of all analyses of low-molecular-weight samples are carried out using RP-HPLC. Dispersive forces employed in this separation mode are the weakest intermolecular forces, thereby making the overall background interaction energy in the chromatographic system very low compared to other separation techniques. This low background energy allows for distinguishing very small differences in molecular interactions of closely related analytes. Adsorbents employed in this mode of chromatography are porous rigid materials with hydrophobic surfaces. The majority of packing materials used in RP-HPLC are chemically modified porous silica.
Adsorption chromatography
The analyte interact with solid stationary surface and are displaced with eluent for active sites on surface.
Partition chromatography
This method results from a thermodynamic distribution of analytes between two liquid phases. On the basis of relative polarities of stationary and mobile phase, partition chromatography can be divided in to normal phase and reverse phase chromatography. In normal phase chromatography, the stationary phase bed is strongly polar in nature (e.g. silica gel) and the mobile phase is non polar (such as n-hexane or tetrahydrofuran). Polar sample are thus retained on polar surface of the column packing longer than polar material while in reverse phase chromatography, the stationary bed is non polar (hydrophobic in nature, while the mobile phase is polar liquid, such as mixture of water and methanol or acetonitrile).
Size exclusion chromatography
This involves a solid stationary phase with controlled pole size. Solids are separated according to molecular size, with the large molecule unable to enter the pores eluted first.
Ion exchange chromatography (IEC)
IEC is based on the differences in affinities of the analyte ions for the oppositely charged ionic center in the resin or adsorbed counter ions in the hydrophobic stationary phase. Consider the exchange of two ions A+ and B+ between the solution and exchange resin
E−: A•E + B+ ↔B•E + A+
The equilibrium constant for this process is shown in Eq. below
K = ([A+][BE])/([AE][B+])
This essentially determines the relative affinity of both cations to the exchange centres on the surface. If the constant is equal to 1, no discriminating ability is expected for this system. The higher the equilibrium constant (provided that, it is greater than 1), the greater the ability of action B+ to substitute A on the resin surface. Depending on the charge of the exchange centres on the surface, the resin could be either anion-exchanger (positive ionic centers on the surface) or cation-exchanger (negative centres on the surface). Cross linked styrene-divinyl benzene is the typical base material for ion exchange resin. Exchange groups are attached to the Phenyl rings in the structure and the degree of cross linkage is between 5 % and 20 %. The higher the cross linkage, the harder the material and the less susceptible it is to swelling, but the material usually shows lower ion-exchange capacity. Four major types of ion- exchange centres are usually employed:
• SO3-—strong cation-exchanger
• CO2-—weak cation-exchanger
• Quaternary amine—strong anion-exchanger
• Tertiary amine—weak anion-exchanger
Analyte retention and selectivity in ion exchange chromatography are strongly dependent on the pH and ionic strength of the mobile phase.
Size exclusion chromatography (SEC)
SEC is the method for dynamic separation of molecules according to their size. The separation is based on the exclusion of the molecules from the porous space of packing material due to their steric hindrance. Hydrodynamic radius of the analyte molecule is the main factor determining its retention. This is the only chromatographic separation method where any positive interaction of the analyte with the stationary phase should be avoided.
In SEC, the higher the molecular weight of the molecule, the greater its hydrodynamic radius results in faster elution. At the same time, if an analyte molecule interacts (undesired) with the stationary phase, thus increasing the retention of larger molecules, which may conform separation of molecules based solely on their hydrodynamic radius. The adsorbent pore size distribution plays the dominant role in the adsorbent ability to discriminate molecules according to their molecular weight. Hydrodynamic radius of the polymer is also dependent on the analyte interaction with the solvent. Polymer conformation and degree of the salvation varies with the variation of the solvent properties.
Instrumentation of hplc
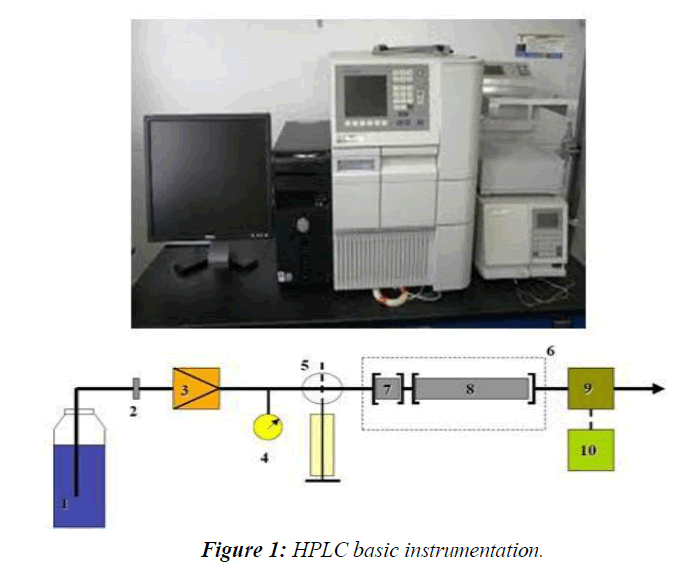
HPLC is a special branch of Column Chromatography in which the mobile phase is forced through the column at high speed. As a result, the analysis time is reduced by 1-2 orders of magnitude relative to classical Column Chromatography and the use of much smaller particles of the absorbent or support becomes possible increasing the column efficiency substantially. The Basic HPLC Instrumentation was shown in the (Figure 1)
Solvent delivery system
The most important component of HPLC in solvent delivery system is the pump, because its performance directly effects the retention time, reproducibility and detector sensitivity. Among the several solvent delivery systems, (direct gas pressure, pneumatic intensifier, reciprocating etc.) reciprocating pump with twin or triple pistons is widely used, as this system gives less baseline noise, good flow rate reproducibility etc.
The pumping systems used in HPLC can be categorized in three different ways.
➢ The first classification is according to the eluent flow rate that the pump is capable of delivering.
➢ The second classification is according to the construction materials.
➢ The final classification is according to the mechanism by which the pump delivers the eluent.
Each of these classifications is considered below.
Pump classification according to flow rate
When classified in terms of flow rate, pumps may be defined as micro bore or preparative.
➢ Standard bore systems are the most commonly used pumping systems for analytical HPLC because they provide reliable operation at flow rates ranging from 100 µl / min to 10 µl / min.
➢ Micro bore systems are intended for use with column diameters ranging up to 2 mm. The narrow column diameter and small size of the packing material causes relatively low flow rates for the pumping system, from 1 to 250 µl / min as the minimum head size for reciprocating pumps is around 25 µl, smooth, reliable operation at flow rates less than 10 µl / min is difficult.
Pump classification according to materials of construction
Pumps may also be classified according to the primary construction materials. The pumps are classified as
➢ Metallic
➢ Non-metallic, depending on the material used for the eluent flow path.
➢ The most commonly used material for HPLC pumping systems is 316 stainless steel, because of its mechanical strength, corrosion resistance, good thermal stability and malleability. Only a handful of HPLC solvents such as Hydrochloric acid will cause damage to 316 stainless steel. Therefore pumps are also constructed from non-metallic materials such as PEEK (poly ethyl ethyl ketone), Teflon (poly tetra fluoro ethylene) and Ceramics.
Pump classification according to mechanism of eluent displacement
The third classification of pumps is according to the mechanism by which the liquid is forced through the chromatograph. The pumps are classified into two types. They are
➢ syringe pumps and
➢ reciprocating-piston pump
HPLC systems are also provided an online degassing system which continuously removes the dissolved gases from the mobile phase.
Solvent degassing system
The constituents of the mobile phase should be degassed and filtered before use. Several methods can be applied to remove the dissolved gases in the mobile phase. They include
➢ heating and stirring,
➢ vacuum degassing with an aspirator,
➢ filtration through 0.45μm filters,
➢ vacuum degassing with an air-soluble membrane,
➢ Helium purging ultra signification or purging or combination of these methods.
Sample introduction system
Two means for analyte introduction on the column are injection into a flowing stream and a stop flow injection. These techniques can be used with a syringe or an injection valve. Automatic injector is a microprocessor-controlled version of the manual universal injector.
Injector
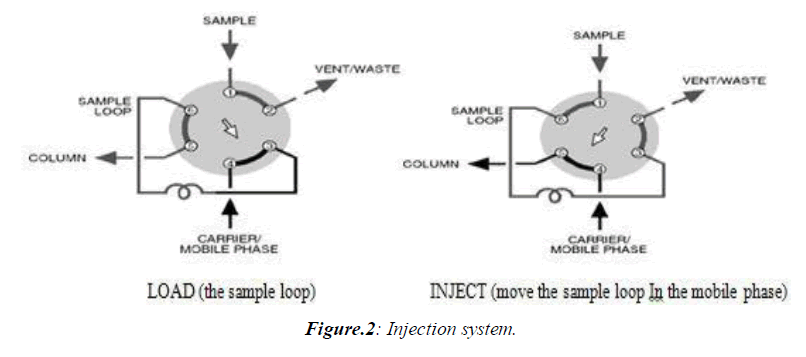
Injectors should provide the possibility of injecting the liquid sample within the range of 0.1 to 100 ml of volume with high reproducibility and under high pressure (up to the 4000 psi). They should also produce minimum band broadening and minimize possible flow disturbances. The most useful and widely used sampling device for modern LC is the micro sampling injector valve. With these sampling valves, samples can be introduced reproducibly into pressurized columns without significant interruption of flow even at elevated temperatures. (Figure 2)
Columns
The heart of the system is the column. Analytical column is the most important part of the HPLC which decides the efficiency of separation. The choice of common packing material and mobile phases depends on the physical properties of the drug.
The following properties of the column stationary phases play an important role in giving different selectivity for separations.
1. Particle size
2. Particle shape
3. Pore size / Pore volume
4. Specific surface area,
5. End capping vi) % carbon loading
The following are the most widely used columns with stationary phases for separation and quantification of wide variety of drugs.
1. Pure silica and hybrid silica columns.
2. Silica based columns with different bonding phases like C4, C6, C8, C18, C20 and bonding phases having functional groups like cyano, phenyl, naphthyl and amino.
3. Silica based columns with polar embedded phases within chains of C8, C18, NH2.
4. Hybrid silica based columns like C4, C6, C8, C18, C20 and bonding phases having functional groups like cyano, phenyl, naphthyl and Amino.
5. Strong cation exchange (SCX) and strong anion exchange (SAX) columns.
6. Size Exclusion chromtography (SEC) or gel permeation chromatography (GPC) columns.
7. Silica based monolith columns.
8. Fused core silica columns with bonding phases like C8, C18, CN, phenyl.
9. Metal oxide columns like zirconia based and alumina based.
10. Chiral columns.
Column-packing materials
Silica (SiO2.X H2O) is the most widely used substance for the manufacture of packing materials it consist of a network of siloxane linkages(Si-O-Si) in a rigid three dimensional structure containing inter connected pores. Thus a wide range of commercial products are available with surface areas ranging from 100 to 800 m2/g and particle sizes from 3 to 50 µm. The silonol groups on the surface of silica give it a polar character, which is exploited in adsorption chromatography using non-polar organic elutents. Silica can be drastically altered by reaction with organochlorosilanes or organoalkoxysilanes giving Si-O-Si-R linkages with the surface. The attachment of hydrocarbon chain to silica produces a non polar surface suitable for reversed phase chromatography where mixtures of water and organic solvents are used as eluents. The most popular material is octa decyl silica (ODS) which contains C18chains, but material with C2, C6, C8 and C22 chains are also available. During manufacture, such materials can be reacted with a small mono functional silane (eg: trimethychlorosilane) to reduce further number of silanol groups remaining on the surface (end capping). There is a vast range of materials which have intermediate surface polarities arising from the bonding to silica of other organic compounds which contain groups such as phenyl, nitro, amino and hydroxyl. Strong ion exchange is also available in which sulphonic acid groups and quaternary ammonium groups are bonded to Silica. The useful pH range for columns is 2 to 8, since Siloxane linkages are cleaved below pH 2 while at pH values above 8 Silica may dissolve. In HPLC, generally two types of columns are used, normal phase column and reversed phase column. Using normal phase chromatography, particularly of non-polar and moderately polar drugs can make excellent separation and was originally believed that separation of compounds in mixtures takes place slowly by differential adsorption on a stationary silica phase. However, it now seems that partition plays an important role, with the compounds interacting with the polar silonol groups on the silica or with bound water molecules. While in normal phase, seems the passage of a relatively non-polar mobile phase over a polar stationary phase, reversed phase chromatography is carried out using a polar mobile phase such as methanol, acetonitrile, water, buffer etc. over a non polar stationary phase. A range of stationary phases (C18, C8, -NH2, -CN, -Phenyl etc.) are available and very selective separation can be achieved.
The most popular brands of LC columns are Inertsil, Hypersil, X-terra, X-bridge, Sun- fire, Atlantis, Aquity-BEH, Zorbax, Lichrosphere, Purosphere, Sperisorb, Luna, Kromasil, ACE, YMC, Symmetry, Chiralcel and Chiralpak. These LC columns are supplied in different dimensions, viz., lengths of 10 mm, 50 mm, 100mm, 150mm, 250mm, 300mm, 500mm and internal diameters of 2.1mm, 3.0mm, 4.0mm, 4.6mm. LC columns with stationary phases having different particle sizes like 5.0 µm, 4.0 µm 3.5 µm, 3.0 µm, 2.5 µm, 2.0 µm, 1.9 µm, 1.8 µm, 1.7 µm and 1.3 µm are available.
Mobile phase
Mobile phases used for HPLC are typically mixtures of organic solvents and water or aqueous buffers.
The following points should also be considered when choosing a mobile phase:
➢ It is essential to establish that the drug is stable in the mobile phase for at least the duration of the analysis.
➢ Excessive salt concentrations should be avoided. High salt concentrations can result in precipitation which can damage HPLC equipment. Reduce cost and toxicity of the mobile phase by using methanol instead of acetonitrile whenever possible.
➢ Minimize the absorbance of buffer. Since trifluroacetic acid or formic acid absorb at shorter wavelengths. They may prevent detection of products without chromophores above 220 nm. Carboxylic acid modifiers can be frequently replaced by phosphoric acid which does not absorb above 200 nm. (Table 2)
| 7 | MW | BP | RI (25°C) | UV Cut-off (nm) | Density g / ml (25°C) | Viscosity cP (25°C) | Dielectric |
|---|---|---|---|---|---|---|---|
| Acetonitrile | 41 | 82 | 1.342 | 190 | 0.787 | 0.358 | 38.8 |
| Dioxane | 88.1 | 101 | 1.42 | 215 | 1.034 | 1.26 | 2.21 |
| Ethanol | 46.1 | 78 | 1.359 | 205 | 0.789 | 1.19 | 24.5 |
| Ethyl acetate | 88.1 | 77 | 1.372 | 256 | 0.901 | 0.45 | 6.02 |
| Methanol | 32 | 65 | 1.326 | 205 | 0.792 | 0.584 | 32.7 |
| CH2Cl2 | 84.9 | 40 | 1.424 | 233 | 1.326 | 0.44 | 8.93 |
| Isopropanol | 60.1 | 82 | 1.375 | 205 | 0.785 | 2.39 | 19.9 |
| n-propanol | 60.1 | 97 | 1.383 | 205 | 0.804 | 2.2 | 20.3 |
| THF | 72.1 | 66 | 1.404 | 210 | 0.889 | 0.51 | 7.58 |
| Water | 18 | 100 | 1.333 | 170 | 0.998 | 1 | 78.5 |
Table 2: Physical properties of common HPLC solvents
Detectors
When a chromophore is present, the wavelength of detection for a drug should be based on its UV Spectrum in the mobile phase and not in pure solvents. The most selective wavelength for detecting a drug is frequently the longest wavelength maximum to avoid interference from solvents, buffers and excipients. Other methods of detection can be useful are required in some instances. The detection of UV light absorbance offers both convenience and sensitivity for molecules.
• Solute specific detectors (UV-Vis, fluorescence, electrochemical, infra-red, radio activity)
• Bulk property detectors (refractive index, viscometer, conductivity)
• Desolvation detectors (flame ionization etc.)
• LC-MS detectors
• Detectors
Applications of HPLC in pharmaceutical research
1. Separation: This can be accomplished using HPLC by utilizing the fact that, certain compounds have different migration rates given a particular column and mobile phase. The extent or degree of separation is determined by the choice of stationary phase and mobile phase along with parameters like flow, temperature and gradient programme.
2. Identification: For this purpose a clean peak of known sample has to be observed from the chromatogram. Selection of column mobile phase and flow rate matter to certain level in this process. Identification is generally by comparing with reference compound based on retention time and also based on UV-Vis spectra in some cases. Identification can be assured by combining two or more detection methods, where necessary.
3. Quantification: Analyte concentrations are estimated by measuring the responses ( peak areas) known reference standards followed by unknown samples. Quantification of known and unknown components are done by various methods like area normalization method, internal standard method, external standard method and diluted standard method along with relative response factors.
4. Isolation: It refers to the process of isolation and purification of compounds using analytical scale or preparative scale HPLC. Volatile buffers and solvents are preferred choice as mobile phases as it reduces the effort on purification. Solute purity and throughput is the key challenge in isolation and purification processes [10-14].
HPLC theory
System suitability parameters
High performance liquid chromatography is defined as a separation of mixtures of compounds due to differences in their distribution equilibrium between two phases, the stationary phase packed inside columns and the mobile phase, delivered through the columns by high pressure pumps. Components whose distribution into the stationary phase is higher, are retained longer, and get separated from those with lower distribution into the stationary phase. The theoretical and practical foundations of this method were laid down at the end of 1960s and at the beginning of 1970s. The theory of chromatography has been used as the basis for system- suitability tests, which are set of quantitative criteria that test the suitability of the chromatographic system to identify and quantify drug related samples by HPLC at any step of the pharmaceutical analysis.
Retention time (tR), capacity factor k' and relative retention time (RRT)
The time elapsed between the injection of the sample components into the column and their detection is known as the retention time (tR). The retention time is longer when the solute has higher affinity to the stationary phase due to its chemical nature. For example, in reverse phase chromatography, the more lyophilised compounds are retained longer.
Therefore, the retention time is a property of the analyte that can be used for its identification. A non-retained substance passes through the column at a time t0, called the void time.
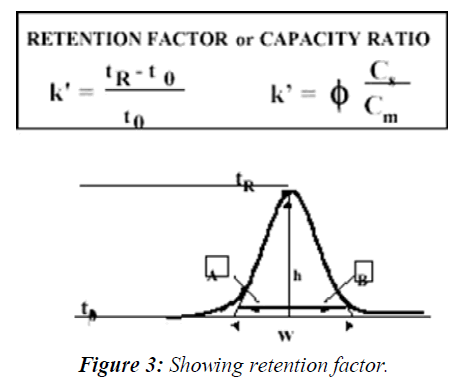
Retention factor is calculated as follows (Figure 3)
The capacity factor describes the thermodynamic basis of the separation and its definition is the ratio of the amounts of the solute at the stationary and mobile phases within the analyte band inside the chromatographic column: Where Cs is the concentration of the solute at the stationary phase and Cm is its concentration at the mobile phase and phi is the ratio of the stationary and mobile phase volumes all within the chromatographic band. The Retention Factor is used to compare the retention of a solute between two chromatographic systems, normalizing it to the column's geometry and system flow rate. The retention factor value should be in between 1-20. The need to determine the void time can be tricky sometimes, due to the instability of the elution time of the void time marker, t0, therefore, when the chromatogram is complex in nature, and one known component is always present at a certain retention time, it can be used as a retention marker for other peaks. In such cases the ratio between the retention time of any peak in the chromatogram and the retention time of the marker is used (tR (Peak) / tR (Marker)) and referred to as the Relative Retention Time (RRT). RRT is also used instead of the capacity ratio for the identification of the analyte as well as to compare its extent of retention in two different chromatographic systems. The sharpness of a peak relative to its retention time is a measure of the system's efficiency, calculated as N, plate count. Band-broadening phenomena in the column such as eddy diffusion, molecular diffusion, and mass-transfer kinetics and extra-column effects reduce the efficiency of the separation. The sharpness of a peak is relevant to the limit of detection and limit of quantification of the chromatographic system. The sharper the peak for a specific area, the better is its signal-to-noise; hence the system is capable of detecting lower concentrations. Therefore, the efficiency of the chromatographic system must be established by the system suitability test before the analysis of low concentrations that requires high sensitivity of the system, such as the analysis of drug impurities and degradation products.
Efficiency
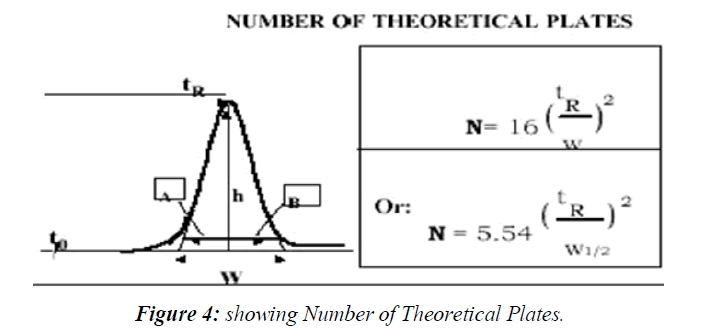
Plate count N and peak capacity Pc
The efficiency of the separation is determined by the plate count N when working at area, the better is its signal-to-noise; hence the system is capable of detecting lower concentrations. Therefore, the efficiency of the chromatographic system must be established by the system suitability test before the analysis of low concentrations that requires high sensitivity of the (Figure 4)
isocratic conditions, whereas it is usually measured by Peak Capacity Pc when working at gradient conditions. The following equation for the plate count is used by the United States
Pharmacopoeia (USP) to calculate N
Where w is measured from the baseline peak width calculated using lines tangent to the peak width at 50 % height. European and Japanese pharmacopoeias use the peak width at 50% of the peak height, hence the equation becomes.
Peak capacity Pc is defined as number of peaks that can be separated within a retention window for a specific pre-determined resolution. In other words, it is the runtime measured in peak width units (34). It is assumed that peaks occur over the gradient chromatogram. Therefore, peak capacity can be calculated from the peak widths in the chromatogram as follows:

Where n is the number of peaks at the segment of the gradient selected for the calculation, tg. Thus peak capacity can be simply the gradient run time divided by the average peak width. The sharper the peaks the higher is the peak capacity, hence the system should be able to resolve more peaks at the selected run time as well as detect lower concentrations.
Another measure of the column's chromatographic efficiency is the height equivalent to theoretical plate (HETP) which is calculated from the following equation:
HETP = (L/N)
Where L is column length and N is the plate count. HETP is measured in micrometer.
The behaviour of HETP as function of linear velocity has been described by various equations. It is frequently called "The Van-Deemter curve", and it is frequently used to describe and characterize various chromatographic stationary phases' performance and compare them to each other. The lower are the values of HETP, the more efficient is the chromatographic system, enabling the detection of lower concentrations due to the enhanced signal-to-noise ratio of all the peaks in the chromatogram.
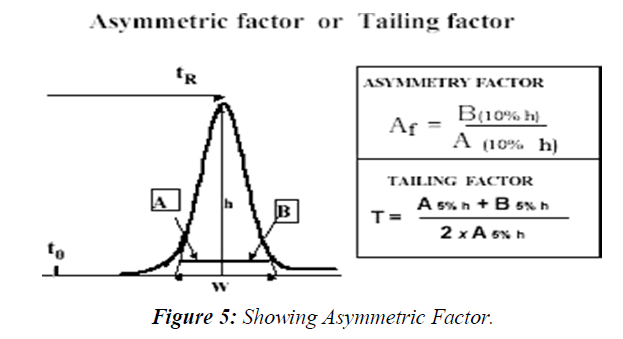
Peak asymmetry factor Af and tailing factor T
The chromatographic peak is assumed to have a Gaussian shape under ideal conditions, describing normal distribution of the velocity of the molecules populating the peak zone migrating through the stationary phase inside the column. Any deviation from the normal distribution indicates non-ideality of the distribution and the migration process therefore might jeopardize the integrity of the peak's integration, reducing the accuracy of the quantitation. This is the reason why USP Tailing is a peak's parameter almost always measured in the system suitability step of the analysis.
The deviation from symmetry is measured by the asymmetry factor, Af or tailing factor T. The calculation of asymmetry factor, Af is described by the following equation (Figure 5)
Where A and B are sections in the horizontal line parallel to the baseline, drawn at 10% of the peak height. The calculation of tailing Factor, T, which is more widely used in the pharmaceutical industry, as suggested by the pharmacopeia’s, where A and B are sections in the horizontal line parallel to the baseline, drawn at 5% of the peak height. The USP suggests that tailing factor should be in the range of 0.5 up to 2 to assure a precise and accurate quantitative measurement.
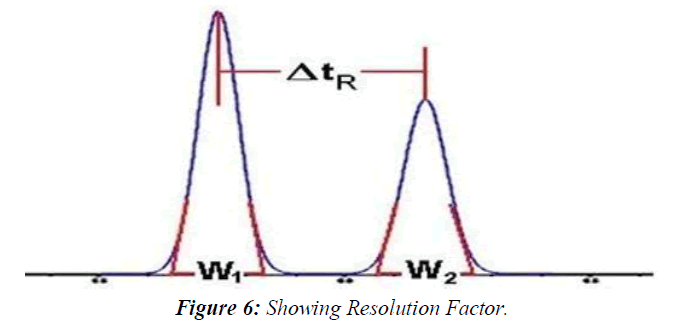
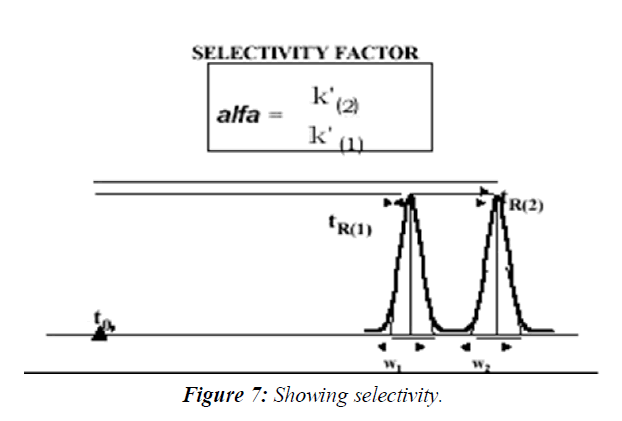
Selectivity Factor α, and Resolution Factor Rs
The separation is a function of the thermodynamics of the system. Substances are separated in a chromatographic column when their rate of migration differs, due to their different distribution between the stationary and mobile phases. The selectivity factor, α, and resolution factor, Rs, measure the extent of separation between two adjacent peaks. The selectivity factor accounts only for the ratio of the retention factors, k', of the two peaks (k'2/k'1), whereas the resolution factor, Rs, accounts for the difference between the retention times of the two peaks relative to their width.(Figure 6)
The equation that describes the experimental measurement of the resolution factor, Rs, is as follows:
Rs = ΔtR / 0.5 (W1 + W2)
Where tR is the retention time of peaks 1 and 2 respectively and w is their respective peak width at the tangents' baseline. According to the pharmacopeia should be above 1.5 for an accurate quantitative measurement.
The resolution is a critical value when working with complex samples such as drug impurities and degradation products, or when the formulation is complex and excipients might interfere with the quantitative measurements. Therefore, it is an essential part of the system suitability measurement stage before the quantitative work of these types of samples. The sample used for the measurements of Rs during the system suitability runs is sometimes called Resolution Solution, It usually contains the components that are the most difficult to resolve. The theoretical description of the Resolution Factor Rs equation is shown in Equation. It includes some of the above parameters, the plate count N, the selectivity α and the average of the two peaks' capacity factors k':( Figure 7)
It can be clearly seen from this equation that the plate count is the most effecting parameter in the increase of the chromatographic resolution. Since the plate count increases with the reduction in particle diameter, it explains the reduction in particle diameter of the stationary phase material during the last 3 decades of HPLC. This is also the rational behind the recent trend in HPLC, the use of sub 2 micron particle columns and the development of a specially design of ultra performance HPLC systems to accommodate such columns.
Analytical method development
Methods are developed for new products when no official methods are available. Alternate methods for existing (Non-Pharmacopoeias) products are developed to reduce the cost and time for better precision and ruggedness. Trial runs are conducted, method is optimized and validated. When alternate method proposed is intended to replace the existing procedure, comparative laboratory data including merits / demerits should be made available.
Steps involved in method development
Documentation starts at the very beginning of the development process. A system for full documentation of development studies must be established. All data relating to these studies must be recorded in laboratory notebook or an electronic database.
Analyte standard characterization
1. All known information about the analyte and its structure is collected i.e., physical and chemical properties.
2. The standard analyte (100 % purity) is obtained. Necessary arrangement is made for the proper storage (refrigerator, desiccators and freezer).
3. When multiple components are to be analyzed in the sample mLinagliptinix, the number of components is noted, data is assembled and the availability of standards for each one is determined.
4. Only those methods (spectroscopic, MS, GC, HPLC etc.,) that are compatible with sample stability are considered.
Method requirements
The goals or requirements of the analytical method that need to be developed are considered and the analytical figures of merit are defined. The required detection limits, selectivity, linearity, range, accuracy and precision are defined.
Literature search and prior methodology
The literature for all types of information related to the analyte is surveyed. solubility profile (solubility of Drug in different solvents and at different pH conditions), analytical profile (Physico-chemical properties, Eg: pKa, melting point, degradation pathways, etc) and stability profile (sensitivity of the drug towards light, heat, moisture etc) and relevant analytical methods, books, periodicals, chemical manufacturers and regulatory agency compendia such as USP / NF, are reviewed.
Choosing a method
1. Using the information in the literatures and prints, methodology is adapted. The methods are modified wherever necessary. Sometimes it is necessary to acquire additional instrumentation to reproduce, modify, improve or validate existing methods for in-house analyses and samples. If there are no prior methods for the analyte in the literature, from analogy, the compounds that are similar in structure and chemical properties are investigated and are worked out.
2. There is usually one compound for which analytical method already exist that is similar to the analyte of interest.
Instrumental setup and initial studies
The required instrumentation is setup. Installation, operational and performance qualification of instrumentation using laboratory standard operating procedures (SOP’s) are verified. Always new consumables (e.g. solvents, filters and gases) are used. For example, method development is never started on a HPLC column that has been used earlier. The analyte standard in a suitable injection / introduction solution and in known concentrations and solvents are prepared. It is important to start with an authentic, known standard rather than with a complex sample mLinagliptinix. If the sample is extremely close to the standard (e.g., bulk drug), then it is possible to start work with the actual sample.
Optimization
During optimization one parameter is changed at a time and set of conditions are isolated, rather than using a trial and error approach. Work has been done from an organized methodical plan, and every step is documented (in a lab notebook) in case of dead ends.
Documentation of analytical figures of merit
The originally determined analytical figures of merit are limit of quantitation (LOQ), limit of detection (LOD), linearity, time per analysis, cost, sample preparation etc., are documented.
Evaluation of method development with actual samples
The sample solution should lead to unequivocal, absolute identification of the analyte peak of interest apart from all other mLinagliptinix components.
Determination of percent recovery of actual sample and demonstration of quantitative sample analysis
Percent recovery of spiked, authentic standard analyte into a sample mLinagliptinix that is shown to contain no analyte is determined. Reproducibility of recovery (average + / - standard deviation) from sample to sample and whether recovery has been optimized or not has been shown. It is not necessary to obtain 100 % recovery as long as the results are reproducible and known with a high degree of certainty. The validity of analytical method can be verified only by laboratory studies.
Therefore documentation of the successful completion of such studies is a basic requirement for determining whether a method is suitable for its intended applications.
Method development procedure
The wide variety of equipment’s, columns, eluent and operation preparations involved high performance liquid chromatography (HPLC) method development seems complex. The processes influenced by the nature of analytes and generally follow the following steps
Steps
• Step 1 - Selection of the HPLC method and initial system
• Step 2 - Selection of initial conditions
• Step 3 - Selectivity optimization
• Step 4 - System optimization
• Step 5 - Method validation.
Depending on the overall requirements and nature of the sample and analytes, some of these steps will not be necessary during HPLC analysis. For example, a satisfactory separation may be found during step 2, thus steps 3 and 4 may not be required. The extent to which method validation (step 5) is investigated will depend on the use of the end analysis; for example, a method required for quality control will require more validation than one developed for a one-off analysis. The following must be considered when developing an HPLC method:
HPLC method development
Step 1 - selection of the HPLC method and initial system
When developing an HPLC method, the first step is always to consult the literature to ascertain whether the separation has been previously performed and if so, under what conditions - this will save time doing unnecessary experimental work. When selecting an HPLC system, it must have a high probability of actually being able to analyse the sample; for example, if the sample includes polar analytes then reverse phase HPLC would offer both adequate retention and resolution, whereas normal phase HPLC would be much less feasible. Consideration must be given to the following:
Sample preparation
• Does the sample require dissolution, filtration, extraction, preconcentration or clean up,
• Is chemical derivatization required to assist detection sensitivity or selectivity
Types of chromatography
1. Reverse phase is the choice for the majority of samples, but if acidic or basic analytes are present then reverse phase ion suppression (for weak acids or bases) or reverse phase ion pairing (for strong acids or bases) should be used. The stationary phase should be C18 bonded.
2. For low/medium polarity analytes, normal phase HPLC is a potential candidate, particularly if the separation of isomers is required. Carbon bonded phases are easier to work with than plain silica for normal phase separations. For inorganic anion/cation analysis, ion exchange chromatography is best. Size exclusion chromatography would normally be considered for analysing high molecular weight compounds.
Column dimensions
For most samples (unless they are very complex), long columns (25 cm) are recommended to enhance the column efficiency. A flow rate of 1-1.5 ml/min should be used initially. packing particle size should be 3 or 5 μm.
Detectors
• Consideration must be given to the following:
• Do the analytes have chromophores to enable UV detection
• Is more selective/sensitive detection required
• What detection limits are necessary
• Will the sample require chemical derivatization to enhance detectability and/or improve the chromatography.
Fluorescence or electrochemical detectors should be used for trace analysis. For preparative HPLC, refractive index is preferred because it can handle high concentrations without over loading the detector. UV wavelength for the greatest sensitivity λmax should be used, which detects all sample components that contain chromophores. UV wavelengths below 200 nm should be avoided because detector noise increases in this region. Higher wavelengths give greater selectivity. The excitation wavelength locates the excitation maximum; that is, the wavelength that gives the maximum emission intensity. The excitation is set to the maximumvalue then the emission is scanned to locate the emission intensity. Selection of the initial system could, therefore, be based on assessment of the nature of sample and analytes together with literature data, experience, expert system software and empirical approaches.
Step 2 - selection of initial conditions
This step determines the optimum conditions to adequately retain all analytes; that is,ensures no analyte has a capacity factor of less than 0.5 (poor retention could result in peak overlapping) and no analyte has a capacity factor greater than 10–15 (excessive retention leads to long analysis time and broad peaks with poor detectability). Selection of the following is then required.
Mobile phase solvent strength
The solvent strength is a measure of its ability to pull analytes from the column. It is generally controlled by the concentration of the solvent with the highest strength; for example, in reverse phase HPLC with aqueous mobile phases, the strong solvent would be the organic modifier; in normal phase HPLC, it would be the most polar one. The aim is to find the correct concentration of the strong solvent. With many samples, there will be a range of solvent strengths that can be used within the aforementioned capacity limits. Other factors (such as pH and the presence of ion pairing reagents) may also affect the overall retention of analytes.
Step 3 - selectivity optimization
The aim of this step is to achieve adequate selectivity (peak spacing). The mobile phase and stationary phase compositions need to be taken into account. To minimize the number of trial chromatograms involved, only the parameters that are likely to have a significant effect on selectivity in the optimization must be examined. To select these, the nature of the analytes must be considered. Once the analyte types are identified, the relevant optimization parameters may be selected. Note that the optimization of mobile phase parameters is always considered first as this is much easier and convenient than stationary phase optimization.
Step 4 - system parameter optimization
This is used to find the desired balance between resolution and analysis time after satisfactory selectivity has been achieved. The parameters involved include column dimensions, column-packing particle size and flow rate. These parameters may be changed without affecting capacity factors or selectivity.
Step 5 - Method validation
Proper validation of analytical methods is important for pharmaceutical analysis when ensure of the continuing efficacy and safety of each batch manufactured relies solely on the determination of quality. The ability to control this quality is dependent upon the ability of the analytical methods, as applied under well-defined conditions and at an established level of sensitivity, to give a reliable demonstration of all deviation from target criteria.
Analytical methods should be used within good manufacturing practice (GMP) and good laboratory practice (GLP) environments, and must be developed using the protocols set out in the international conference on harmonization (ICH) guidelines (Q2A and Q2B). The US food and drug administration (FDA) and US Pharmacopoeia (USP) both refer to ICH guidelines. The most widely applied validation characteristics are accuracy, precision (repeatability and intermediate precision), specificity, detection limit, quantitation limit, linearity, range, robustness and stability of analytical solutions. Method validation must have a written and approved protocol prior to use.
Stability indicating method
It is essential that the analytical methods developed for estimation of the purity and impurities are capable enough to separate all the desired and undesired components and devoid of any interferences from the formulation mLinagliptinix. When analytical methods are able to precisely and accurately quantify without missing any impurities, without underestimation or over estimation, and detect all possible impurities and degradants those can form during stability studies with adequate sensitivity and exactly reflect the quality of drug substances and drug products (formulated products of drugs), those methods are called stability indicating methods.
A stability-indicating assay method should accurately measure the active ingredients, without interference from degradation products, process impurities, excipients, or other potential impurities. If an industry uses a non-stability indicating analytical procedure for release testing, then an analytical procedure capable of qualitatively and quantitatively monitoring the impurities, including degradation products, should complement it. Analytical procedures for stability studies of assay should be stability indicating. As a result of stability testing a re- test period for the active substance or a shelf life for the pharmaceutical product can be established, and storage conditions can be recommended.
The ICH (International conference on Harmonization) guideline QIA on Stability Testing of New Drug Substances and Products emphasizes that the testing of those features which are susceptible to change during storage and are likely to influence quality, safety and/or efficacy must be done by validated stability indicating testing methods. It is also mentioned that forced decomposition studies (stress testing) at temperatures in 10 °C increments above the accelerated temperatures, extremes of pH, under oxidative and photolytic conditions should be carried out on the drug substance and drug product so as to establish the inherent stability characteristics and degradation pathways to support the suitability of the proposed analytical procedures.
Analytical method validation
According to ICH Guidelines Method Validation can be defined as “Establishing documented evidence, which provides a high degree of assurance that a specific activity will consistently produce a desired result or product meeting its predetermined specifications and quality characteristics”.
An assay for a major component requires a different approach and acceptance criteria than a method for a trace impurity. A final method may be performed at different sites around the world. Differences in HPLC instrumentation, laboratory equipment and reagent sources and variations in the skills and background of personnel may require specific features in the HPLC method. In addition, the development of different formulations of the same drug with varying strengths or physical forms may require flexibility in method procedures.
Method validation study include system suitability, linearity, precision, accuracy, specificity, robustness, limit of detection, limit of quantification and stability of samples, reagents, instruments.
System Suitability
Prior to the analysis of samples of each day, the operator must establish that the HPLC system and procedure are capable of providing data of acceptable quality. This is accomplished with system suitability experiments, which can be defined as tests to ensure that the method can generate results of acceptable accuracy and Precision. The requirements for system suitability are usually developed after method development and validation have been completed. (Table 3)
| Parameter | Recommendation |
|---|---|
| Capacity Factor (K’) | The peak should be well-resolved from other peaks and the void volume generally K>2 |
| Repeatability | RSD ≤ 2% |
| Relative Retention | Not essential as the resolution is stated |
| Resolution(Rs) | Rs of > 2 between the peak of interest and the closest eluting |
| Tailing Factor(T) | T ≤ 2 |
| Theoretical Plates(N) | In general should be > 2000 |
Table 3: System Suitability Parameters and their recommended limits
Linearity
The linearity of a method is a measure of how well a calibration plot of response vs. concentration approximates a straight line. Linearity can be assessed by performing single measurements at several analyte concentrations. The data is then processed using a linear least-squares regression. The resulting plot slope, intercept and correlation coefficient provide the desired information on linearity.
Precision
Precision can be defined as “The degree of agreement among individual test results when the procedure is applied repeatedly to multiple samplings of a homogenous sample”. A more comprehensive definition proposed by the International Conference on Harmonization (ICH) divides precision into three types:
1. Repeatability
2. Intermediate precision and
3. Reproducibility
Repeatability is the precision of a method under the same operating conditions over a short period of time.
Intermediate precision is the agreement of complete measurements (including s
References
- Sharma BK. Instrumental methods of chemical analysis. Krishna Prakashan Media; 1981.
- Lindholm J. Development and validation of HPLC methods for analytical and preparative purposes Doctoral dissertation, Acta Universitatis Upsaliensis.
- Rashmin, An introduction to analytical Method Development for Pharmaceutical formulations. Indo J Pharm sci . 2012 ;2 (2): 191-196.
- Malviya R, Bansal V, Pal OP, et al. High performance liquid chromatography: A short review. J Global pharm tech. 2010;2(5):22-6.
- Dr.S. Ravi Shankar. Text book of Pharmaceutical analysis, Fourth edition : 13.1- 13.2
- David G, Watson. Pharmaceutical Analysis, A text book for Pharmacy students and Pharmaceutical Chemists. Harcourt Publish Limit: 221-232.
- Remington JP. Remington: The science and practice of pharmacy. Lippincott Williams & Wilkins; 2006. Indexed at, Google Scholar
- Connors KA. A textbook of pharmaceutical analysis. John Wiley Sons; 2007.
- Sharma BK. Instrumental methods of chemical analysis. Krishna Prakashan Media; 1981.
- Watson DG. A Textbook for Pharmacy Students and Pharmaceutical Chemists. 1999.
- Nasal A, Siluk D, Kaliszan R. Chromatographic retention parameters in medicinal chemistry and molecular pharmacology. Curr Med Chem. 2003;10(5):381-426.
- Kumar A, Kishore L, Kaur N, et al. Method Development and Validation for Pharmaceutical Analysis. Internat Pharmace Sci. 2012;2(3).
- Chandrul Kaushal K, Srivastava B. A process of method development: A chromatographic approach. J Chem Pharm Res. 2010;2(2):519-45.
- Gupta V, Jain AD, Gill NS. Kapil, Development and Validation of HPLC method. Internat Res J Pharmae Applied Sci. 2012;2(4).
Indexed at, Google Scholar, Cross Ref