Case Report - Immunology Case Reports (2023) Volume 6, Issue 2
Reduction of respiratory infections in a patient with profound hypogammaglobulinemia and B-cell chronic lymphocytic leukemia, treated with dialyzable leukocyte extract.
Erika Coria-Ramírez1*, María del Carmen Sánchez-León1, María C. Jiménez Martínez2
1Unidad de Servicios Externos de Investigación Clínica, Instituto Politécnico Nacional, Mexico
2Department of Biochemistry, National Autonomous University of México, México
- *Corresponding Author:
- Erika Coria-Ramírez
Department of External Clinical Research Services Unit (USEIC)
National Polytechnic Institute, 02970, Mexico
E-mail: ecoriar@ipn.mx
Received: 29-Mar-2023, Manuscript No. AAICR-23-90081; Editor assigned: 31-Mar-2023, PreQC No. AAICR -23-90081(PQ); Reviewed: 14-Apr-2023, QC No. AAICR -23-90081; Revised: 19-Apr-2023, Manuscript No. AAICR -23-90081(R); Published: 26-Apr-2023, DOI:10.35841/aaicr-6.2.137
Citation: Coria-Ramírez E. Reduction of respiratory infections in a patient with profound hypogammaglobulinemia and B-cell chronic lymphocyticleukemia, treated with dialyzable leukocyte extract. Immunol Case Rep. 2023;6(2):137
Abstract
B-cell chronic lymphocytic leukemia (B-CLL) is the most frequent leukemia in adults in western countries, it is associated with immune dysfunction at many levels, but humoral immunodeficiency is the most relevant clinical trait. Hypogammaglobulinemia can be present at the diagnosis, but it would worsen due to chemotherapy. Immunoglobulin levels are variable, putting patients at risk of multiple infections. Treatment of hypogammaglobulinemia consists of antimicrobial prophylaxis, vaccines, and human immunoglobulin G replacement. We present the case report of a 67 years-old male patient with B-CLL who received three cycles of fludarabine, cyclophosphamide, and dexamethasone, achieving disease control but developed hypogammaglobulinemia, causing him recurrent upper respiratory tract infections. We initiated treatment with Dialyzable leukocyte extract, an immunomodulator derived from human leukocytes, which has been used for several decades in different immunological diseases. After 24 months of treatment, the patient´s respiratory infections decreased Conclusion: Dialyzable leukocyte extract can be effective in controlling recurrent infections in patients with CLL-associated hypogammaglobulinemia
Keywords
B-cell chronic lymphocytic leukemia, Dialyzable leukocyte extract, Transferon, Hypogammaglobulinemia, Infections, Case report.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is the most frequent leukemia in adults in western countries [1]; CLL is more common in male patients and tends to affect older adults. This disease is caused by the expansion and accumulation of monoclonal, phenotypically mature malignant CD5+ B lymphocytes in the peripheral blood, bone marrow, and lymph nodes [2].
Most B-CLL lymphocytes are arrested at the G0/G1 phase of the cell cycle, as demonstrated by Andreeff M et al. (1988) [3]. Furthermore, Kitada S et al. (1998) [4] described that B-CLL cells have an increased capacity to resist normal apoptotic signals. Thus, the progressive accumulation of B-CLL cells could be a consequence of the prolonged survival of these cells. The International Workshop on CLL guidelines establishes for diagnosis an absolute clonal lymphocyte count of 5000 cells/mm3 in peripheral blood for at least three months. Clonality must be confirmed by cell expression of surface CD19, CD5, and CD23. The expression of CD20 is generally weak, and the malignant cells are either κ or λ light chain restricted [5].
Most patients are asymptomatic at diagnosis; the only sign is leukocytosis and lymphocytosis found at the routine laboratory examination. Therefore, CLL does not require treatment until symptoms develop or the disease progresses, causing severe cytopenia. The major cause of morbidity and mortality in these patients is infections. It has been described that up to 50-60% of deaths are due to infections, mainly bacterial [6]. They occur due to CCL immune dysfunction and the immunosuppressive effects of treatment.
A significant clinical peculiarity of CLL is humoral immunodeficiency. Hypogammaglobulinemia is present at variable degrees and has been observed in more than 50% of the patients with CLL [7], but the IgG subclass deficiency has been reported to be more prevalent. Freeman JA et al. (2013) reported it in 64.6% ofpatients; however, theyfound no consistentcorrelation between immunoglobulin levels and the incidence of infection [8]. More recently, it has been reported that approximately one-quarter of CLL patients have hypogammaglobulinemia at diagnosis, but the risk of developing hypogammaglobulinemia in untreated patients is 11% and 23% at 5 and 10 years, respectively [9]. The severity of hypogammaglobulinemia depends on the stage and duration of the disease.
Other immunologic defects described in CLL patients are excessive T-cell suppression [10] and abnormality in complement function. For example, Fust et al. (1985) [11] found abnormalities in the classical complement pathway with a reduction in mean C1 and C4 levels in >50% of patients tested. However, defects in activating the alternative complement pathway via C3b, which results in the inability to coat bacteria, are perhaps the most frequent [12].
The most common type of infection in these patients is a bacterial infection of the paranasal sinuses and respiratory tract, frequently by encapsulated bacteria; this may be related to deficiencies in serum IgG2 and IgG4 IgA [13]. Further, low levels of specific antibodies against S. pneumoniae, tetanus toxoid, and diphtheria toxoid have been found in correlation with hypogammaglobulinemia and the presence of normal total IgG [14].
In patients with CLL, reactivation of varicella zoster and recurrent attacks of herpes simplex virus are not uncommon. These infections support defective cell-mediated immunity, but significant reductions in serum IgG3 have also been reported [15], which may contribute to the increased risk of these infections.
There is no treatment consensus in CLL patients with hypogammaglobulinemia and recurrent infections. The potential treatment for immunodeficiency includes intravenous immunoglobulin (IVIG), antimicrobial prophylaxis, and vaccination. An early trial reported that using 0.4 g/kg of IVIG every three weeks for one year significantly reduced mild to moderate infections but showed no impact on the rate of severe infections or mortality [16]. More recently, a meta-analysis of randomized controlled trials concluded a significant decrease in severe infections and a significant reduction in clinically documented infections, but no survival benefit could be demonstrated [17]. Also, the high cost of IVIG limits its use.
There are no clinical trials of prophylactic antimicrobial agents in CLL. Although, the use of antibiotics to prevent infections in patients with recurrent sinopulmonary infections is common in routine clinical practice. Observations come from prophylactic antimicrobials in patients with low CD4+ T-cell levels, such as acquired immunodeficiency syndrome (AIDS). CLL patients who received treatment with purine analogs or alemtuzumab have the highest risk of becoming T- cell immunodeficient. There are some published antibiotic prophylaxis recommendations [18].
CLL patients show diminished humoral response to antigen immunization. In general, antibody responses to vaccines are weak. Sinisalo M et al. (2001) [19] reported a higher anti-toxoid antigen response in Binet A stage patients with normal serum immunoglobulin levels. The responses to polysaccharide vaccines observed were 50% to the pneumococcal vaccine and 48% to H. influenzae type b-vaccine in patients with earlier stages of B-CLL, higher gammaglobulin levels and total IgG-levels and 2 and 4 IgG-subclasses [20]. These data suggest that CLL patients should be vaccinated as soon as the diagnosis is made (Table 1).
| Date | Leukocytes | Lymphocytes (1,500-4,500)* | Neutrophils (2,000-7,500)* | Monocytes (200-800)* | Hb (13-18 g/dL)** | Platelets (150,000-400,000)* | Albumin** (3.2-4.6) | Globulin** (2-4) | Albumin/globulin Ratio** (1.0-2.5) |
|---|---|---|---|---|---|---|---|---|---|
| (4,000-11,000)* | |||||||||
| 13-Jun-12 | 82,200 | 75,620 | 2470 | 329 | 16.5 | 132,000 | |||
| 16-Aug-12 | 16,500 | 2,640 | 11,715 | 311 | 16.5 | 139,000 | 4.8 | 1.9 | 2.5 |
| 9-Sep-12 | 5,580 | 893 | 3,962 | 393 | 16.6 | 138,000 | 4.7 | 2.3 | 2 |
| 9-Jan-13 | 6,460 | 646 | 5,233 | 581 | 17.7 | 181,000 | 4.7 | 2.5 | 1.9 |
| 14-Mar-13 | 9,110 | 1,730 | 6,490 | 740 | 17.4 | 277,000 | 4.9 | 2.6 | 1.9 |
| 18-Jun-13 | 11,750 | 1,900 | 8,590 | 920 | 16.8 | 194,000 | 4.6 | 2.4 | 1.9 |
| 10-Jan-14 | 5,520 | 770 | 2,570 | 190 | 17.9 | 163,000 | 4.5 | 2.5 | 1.8 |
| 19-Jan-15 | 8,200 | 3,120 | 4,260 | 200 | 17.3 | 182,000 | 4.6 | 2.4 | 1.9 |
| 15-Apr-16 | 13,560 | 8,360 | 4,210 | 710 | 17.8 | 150,000 | 5.1 | 2.5 | 2 |
| 20-Feb-17 | 15,990 | 9,210 | 5,530 | 970 | 17.3 | 149,000 | 4.8 | 2.1 | 2.3 |
| 12-Jan-18 | 21,220 | 16,030 | 4,150 | 710 | 16.7 | 160,000 | 4.6 | 2.2 | 2.1 |
| 23-Jan-19 | 23,560 | 17,410 | 5,050 | 850 | 16.9 | 158,000 | 4.8 | 1.9 | 2.5 |
| 12-Sep-19 | 23,370 | 17,700 | 4,590 | 790 | 17.1 | 140,000 | 4.8 | 1.3 | 3.7 |
| 11-Nov-19 | 27,030 | 22,010 | 3,880 | 830 | 16.5 | 152,000 | 4.6 | 1.8 | 2.5 |
| 20-Jul-20 | 33,680 | 27,950 | 4,700 | 860 | 16.7 | 118,000 | 4.6 | 1.8 | 2.5 |
| 14-May-21 | 36,000 | 30,960 | 4,000 | 780 | 16.2 | 114,000 | 4.4 | 1.7 | 2.6 |
| 4-Aug-21 | 46,760 | 41,350 | 4,200 | 900 | 16.2 | 127,000 | 4.4 | 1.7 | 2.6 |
** Values are presented in g/dL. (Normal ranges)
Table 1. Laboratory exams.
We present a patient with CLL and hypogammaglobulinemia with recurrent upper respiratory infections who received human dialyzable leukocyte extract (DLE), a peptide-based immunomodulator, to diminish recurrent infections.
Case presentation
A 67 years-old male patient came for immunology consultation on December 17, 2019, complaining of recurrent upper respiratory infections during the past three years. He was a university teacher with no family history of allergy or immunodeficiency. He had systemic arterial hypertension treated with olmesartan/hydrochlorothiazide 40mg/12.5mg QD. Seven years before, he was diagnosed with CLL and classified as Rai II, Binet A stage because he had B symptoms, mild thrombocytopenia, and hepatomegaly 3cm larger than average. In the bone marrow, there were 40% of small and mature-appearance lymphocytes; by immunophenotyping analysis, the cells expressed CD19+, CD20+/-, CD23+, and CD5+. In February 2012, he received Fludarabine 50mg QD, Cyclophosphamide 500mg QD, and Dexamethasone 40mg QD, each for 4 days every 21 days for three cycles, bringing the disease under control. Blood count data are summarized in table 1.
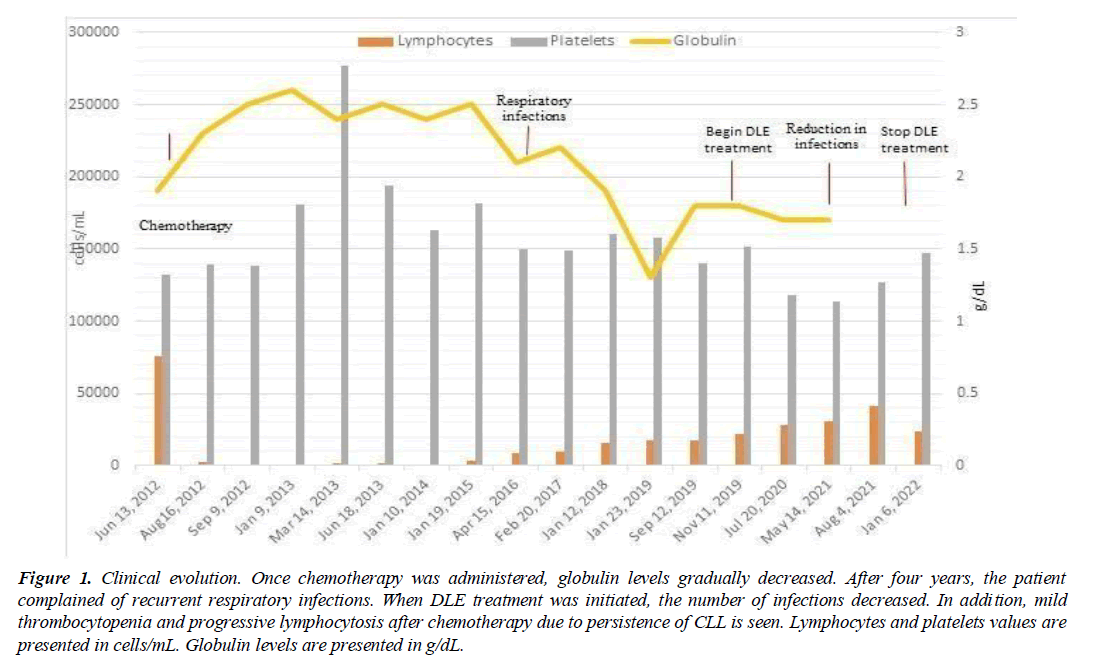
After chemotherapy treatment, the patient was kept under control in the Hematology consultation. However, in April 2016, he presented progressive lymphocytosis and mild thrombocytopenia again. Since then, the patient presented recurrent upper respiratory tract infections with 4-5 episodes per year, characterized by pharyngeal pain, general malaise, and dysphonia, each requiring antimicrobial treatment. The symptoms appeared coincidentally with a decrease in globulin levels, which could be caused by hypogammaglobulinemia, explaining the infection, see (Figure 1).
Figure 1: Clinical evolution. Once chemotherapy was administered, globulin levels gradually decreased. After four years, the patient complained of recurrent respiratory infections. When DLE treatment was initiated, the number of infections decreased. In addition, mild thrombocytopenia and progressive lymphocytosis after chemotherapy due to persistence of CLL is seen. Lymphocytes and platelets values are presented in cells/mL. Globulin levels are presented in g/dL.
In the immunology consultation in December 2019, we initiated oral human DLE every 15 days for six months, then every 21 days for 18 months. After eighteen months, the patient presented only one upper respiratory tract infection. In December 2020, hypogammaglobulinemia was confirmed (Table 2). After two years of treatment, the immunomodulator was stopped. By January 2022 the patient was still respiratory infections-free.
| Date | IgG (540-1822) | IgA (101-645) | IgM (22-240) |
|---|---|---|---|
| 13-Dec-20 | 473.85 | 41.95 | 5.32 |
| 14-May-21 | 447.68 | 41.45 | 5.22 |
Table 2. Immunoglobulin values.
Discussion
Hypogammaglobulinemia is a common feature in patients with CLL; it can be present at the moment of diagnosis and worsens in more advanced stages of the disease. The major cause of morbidity and mortality in these patients is infections. Up to 50-60% of deaths are due to infections, mainly bacterial [6]. They occur due to immune dysfunction and immunosuppressive effects of treatment.
We presented a clinical case of 67 years-old male with CLL; he received three chemotherapy cycles going into disease control; four years after being treated, the patient developed progressive hypogammaglobulinemia and multiple upper respiratory tract infections. We started DLE, a peptide- based immunomodulator, which has been used for several decades in patients with immunodeficiencies, and recurrent and persistent infections [21]. Interestingly, DLE addition improved and diminished respiratory infections.
There is no specific therapy for immunodeficiency in patients with CLL and hypogammaglobulinemia. IVIG has some benefits but is very expensive. Prophylactic antibiotics have specific purposes. Vaccination against infections is generally ineffective in advanced stages of CLL. Human DLEs, are heterogeneous mixtures of low-molecular-weight peptides (<10kDa) obtained from the lysis and dialysis of human buffy coat. [22] The most abundant peptide in Transferon is monomeric ubiquitin (mUb) [23]. Several immunoregulatory properties have been reported for Transferon, such as differential regulation of the production of TNF-α, IL-6, and IL-8 cytokines [24, 25]. These immunoregulatory activities could be explained by the interaction of mUb with their receptor CXCR4 [23]. In humans, DLEs have been used to treat recurrent infections and primary immunodeficiencies, such as Wiskott-Aldrich syndrome, and in sepsis with satisfactory results and good safety profile and tolerability. [26-28] In this context, it has been recognized that extracellular ubiquitin has peptidic sequences with important antimicrobial activity [29].
In this case, the patient showed an acceptable clinical response when adding DLE, without requiring IVIG or antibiotic prophylaxis. Although we observed clinical improvement, there were no changes in immunoglobulin values. Therefore, the reduction in infections can be attributed to the several immunoregulatory mechanisms mentioned above that could activate innate or acquired immune responses.
Finally, this case had several limitations in the clinical evaluation; for example, the immunoglobulin levels at diagnosis of CLL and when infections began were not determined. Additionally, vaccination against pneumococcal and influenza viruses was not performed. However, this case teaches us that using an appropriate biotherapeutic product can offer additional therapeutic support to the patient, favoring the reduction and control of recurrent respiratory infections.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by Instituto Politécnico Nacional
References
- Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105(11):1684-92.
- Nabhan C, Rosen ST. Chronic lymphocytic leukemia: a clinical review. Jama. 2014;312(21):2265-76.
- Andreeff MZ, Darzynkiewicz Z, Sharpless TK, et al. Discrimination of human leukemia subtypes by flow cytometric analysis of cellular DNA and RNA. Blood. 1980;55(2):282-293.
- Kitada S, Andersen J, Akar S, et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with in vitro and in vivo chemoresponses. Blood, Am Soc Hematol. 1998;91(9):3379-89.
- Hallek M. Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2017;92(9):946-65.
- Molica S. Infections in chronic lymphocytic leukemia: risk factors, and impact on survival, and treatment. Leuk Lymphoma. 1994;13(3-4):203-14.
- Itala M, Helenius H, Nikoskelainen J, et al. Infections and serum IgG levels in patients with chronic lymphocytic leukemia. Eur J Haematol. 1992;48(5):266-70.
- Freeman JA, Crassini KR, Best OG, et al. Immunoglobulin G subclass deficiency and infection risk in 150 patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54(1):99-104.
- Parikh SA, Leis JF, Chaffee KG, et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: Natural history, clinical correlates, and outcomes. Cancer. 2015;121(17):2883-91.
- Kay NE. Abnormal T-cell subpopulation function in CLL: excessive suppressor (T gamma) and deficient helper (T mu) activity with respect to B-cell proliferation. 1981;57:418-420.
- Füst G, Czink E, Minh D, et al. Depressed classical complement pathway activities in chronic lymphocytic leukaemia. Clin Exp Immunol. 1985;60(3):489.
- Heath ME, Cheson BD. Defective complement activity in chronic lymphocytic leukemia. Am J Hematol. 1985;19(1):63-73.
- Aittoniemi J, Miettinen A, Lainf S, et al. Opsonising immunoglobulins and mannan-binding lectin in chronic lymphocytic leukemia. Leuk Lymphoma. 1999;34(3-4):381-5.
- Griffiths H, Lea J, Bunch C, et al. Predictors of infection in chronic lymphocytic leukaemia (CLL). Clin Exp Immunol. 1992 ;89(3):374-7.
- Copson ER, Ellis BA, Westwood NB, et al. IgG subclass levels in patients with B cell chronic lymphocytic leukaemia. Leuk Lymphoma. 1994;14(5-6):471-3.
- Gale RP, Chapel HM, Bunch C, et al. Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia. A randomized, controlled clinical trial. N Engl J Med. 1988;319(14):902-7.
- Raanani P, Gafter-Gvili A, Paul M, et al. Immunoglobulin prophylaxis in chronic lymphocytic leukemia and multiple myeloma: systematic review and meta-analysis. Leuk Lymphoma. 2009;50(5):764-72.
- Hilal T, Gea-Banacloche JC, Leis JF. Chronic lymphocytic leukemia and infection risk in the era of targeted therapies: Linking mechanisms with infections. Blood reviews. 2018;32(5):387-99.
- Sinisalo M, Aittoniemi J, Oivanen P, et al. Response to vaccination against different types of antigens in patients with chronic lymphocytic leukaemia. Br J Haematol. 2001;114(1):107-10.
- Hartkamp A, Mulder AH, Rijkers GT, et al. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B-cell chronic lymphocytic leukaemia. Vaccine. 2001;19(13-14):1671-7.
- Berrón-Pérez R, Chávez-Sánchez R, Estrada-García I, et al. Indications, usage, and dosage of the transfer factor. Rev Alerg Mex. 2007;54(4).
- Medina-Rivero E, Vallejo-Castillo L, Vázquez-Leyva S, et al. Physicochemical characteristics of Transferon batches. BioMed Res Int. 2016;2016.
- Vallejo-Castillo L, Favari L, Vázquez-Leyva S, et al. Sequencing analysis and identification of the primary peptide component of the dialyzable leukocyte extract “Transferon Oral”: The starting point to understand its mechanism of action. Front Pharmacol. 2020;11:569039.
- Salinas-Jazmín N, Estrada-Parra S, Becerril-García MA, et al. Herpes murine model as a biological assay to test dialyzable leukocyte extracts activity. J Immunol Res. 2015;2015.
- Robles-Contreras A, Vizuet L, Rivera E, et al. Down regulation of IL-8 and IL-6 in human limbal epithelial cells cultured with human dialyzable leukocyte extracts. Rev Alerg Mex. 2011;58(3).
- Ayala MD, González NM, Palacios G, et al. Dialyzed leukocyte extracts for the treatment of recurrent and severe infections in pediatric patients with cellular immunodeficiency: 15 years of experience. Rev Alerg Mex. 2019;66(1):27-37.
- Castrejón Vázquez MI, Reséndiz-Albor AA, Ynga-Durand MA, et al. Dialyzable leukocyte extract (Transferon™) administration in sepsis: Experience from a single referral pediatric intensive care unit. BioMed Res Int. 2019;2019.
- Homberg T, Sáenz V, Galicia-Carreón J, et al. The adverse event profile in patients treated with Transferon TM (Dialyzable leukocyte extracts): a preliminary report. J Pharm Pharmacol. 2015;6(02):65.
- Kieffer AE, Goumon Y, Ruh O, et al. The N?and C?terminal fragments of ubiquitin are important for the antimicrobial activities. The FASEB J. 2003;17(6):776-8.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref