Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2023) Volume 13, Issue 100
Purification and characterization of lectin from Indian borage leaves (Plectranthus amboinicus)..
Roopashri N. Arekal1, Mohan kumar S2, Keerthana H1, Manoj kumar D3, Muralidhar S1, Priya R1, Vijay kumar CB1, Savitha Janakiraman1*1Department of Microbiology, Biotechnology and Food Technology, Bangalore University, Jnanabharathi campus, Bengaluru, India.
2Department of Food Science and Technology, St Joseph’s University, Bengaluru, India.
3Department of Microbiology, Medopharm Company, Bengaluru, India
- *Corresponding Author:
- Savitha Janakiraman
Department of Microbiology
Biotechnology and Food Technology
Bangalore University, Jnanabharathi campus
Bengaluru, India
E-mail: drsvtj@yahoo.co.in
Received: 27-Jun-2023, Manuscript No.AABPS-23-104820; Editor assigned: 29-Jun-2023, Pre QC No. AABPS -23-104820(PQ); Reviewed: 13-Jul-2023, QC No. AABPS-23-104820; Revised: 17-Jul-2023, Manuscript No. AABPS-23-104820(R); Published: 25-Jul-2023, DOI:10.35841/aabps-13.100.181
Citation: Janakiraman S. Purification and characterization of lectin from Indian borage leaves (Plectranthus amboinicus). Asian J Biomed Pharmaceut Sci 2023; 13(100):181
Abstract
Lectins are glycan-binding proteins that have been of interest to researchers because of their multi-physiological role. The demonstration of the functionality of lectin from Indian borage leaves (Plectranthus amboinicus) with respect to its antioxidant activity is inadequate. In the present study, lectin was extracted and purified from the leaves of Indian borage by affinity chromatography using Seralose-4B beads that were coupled with dextrose sugar. The purified lectin exhibited haemagglutination activity (HA) with chicken erythrocytes, which showed a higher glycan-specificity to dextrose. Purified Plectranthus amboinicus lectin (PAL) exhibited a molecular weight of 19 kDa and 24 kDa by SDS-PAGE, indicating that this haemagglutinin exists as a dimer composed of two subunits of different molecular weights. The effects of different pH and temperature as well as influence of divalent cations on lectin activity were examined. The PAL activity was constant over a broad range of pH and temperature, and its activity was stable in the presence of divalent cations such as Mg2+ and Ca2+. The basic structure of PAL was studied by FTIR, which revealed that its secondary structure contains α-helix structure. Further examination of PAL bioactivity by the DPPH assay showed an IC50 value of 11.44 µg/mL. These results provide insight into the benefits of Indian borage lectin that can be further explored for its therapeutic value.
Keywords
Lectin, Plectranthus amboinicus, Haemagglutination activity, Purification, Characterization, Antioxidant activity.
Introduction
The Plectranthus amboinicus, also known as Coleus amboinicus, is a dicotyledonous plant belonging to the Lamiaceae family having strong oregano-like flavor. It grows up to 1 m tall, and the leaves are tender, fleshy, broad, and oval-shaped. P. amboinicus is considered a native plant of Africa, the Arabian Peninsula, and India, where it is used not only as ornamental plant but also as a spice. It is generally known as Indian borage, country borage, French thyme, Indian mint, Mexican mint, soup mint and so on [1]. It is widely used in Indian medicine. P. amboinicus leaf extract is used for respiratory diseases (such as congestion, bronchitis, sour throat, etc.) and digestive system diseases (dysentery, diarrhoea, colitis, etc.). P. amboinicus leaves have been informed to have many pharmacological properties, such as urolithiasis, antiepileptic, antitumorogenic, antimutagenic, radioprotective, neuropharmacological, and antimicrobial properties [2,3]. Many Lamiaceae plants are aromatic and produce a variety of essential oils along with the other important biological compounds. Most studies on P. amboinicus have focused mainly on the identification and characterization of secondary metabolites such as essential oils, flavonoids, terpenoids, antioxidants, and other compounds with pharmacological activity. These compounds are considered as the main active constituents. The characterization of bio-components is very essential, where lectins are one among them. However, the proteinaceous constituents of P. amboinicus are barely studied so far.
Lectins are heterogeneous proteins of non-immune source and reversibly bind to carbohydrates having more than one non- catalytic domain, resulting in cell agglutination. This means that agglutination is the result of a non-covalent interaction between sugars on the cell surface and binding sites on lectin molecules. They interact with specific glycan structures such as soluble and membrane-bound glycoconjugates, thereby engaging in many kinds of biological functions, mainly cell-cell communication, host defense, fertilization, cell development and differentiation [4-7]. They are a structurally diverse class of proteins that are extensively distributed in nature and found mainly in plants, mushrooms, algae, animals, and micro-organisms. The presence of lectins in plants has been observed in several families, including Rubiaceae [8], Fabaceae (leguminous) [9], Lamiaceae [10], Dioscoreaceae [11], Cucurbitaceae [12] and Arecaceae [13]. So far, there have been no reports of lectins from Plectranthus amboinicus belonging to the Lamiaceae family.
The literature confirms that plant-derived lectins exhibit diverse biological activities, including anti-microbial, anti-nociceptive, anti-inflammatory, mitogenic, anti- fungal and anti-HIV-1 reverse transcriptase activities as well as anticancer properties in human cell lines [14, 15]. They are also involved in the development of symbiotic associations between plants and nitrogen-fixing microbes [16]. Historically, they were used as models to study protein- carbohydrate interactions. They are used as reagents in biochemical, pharmaceutical, and clinical research because of their distinct glycan-binding specificities [17]. They act as indicators of pathological conditions, disease characteristics, stage of disease progression, and cell differentiation [18-20]. They have been used in pharmaceuticals to produce low-cost protein expression systems and nutraceutics. Lectins from terrestrial plants have been widely used as analytical tools in biotechnology and biomedical research, despite the fact that many of their precise biological activities are still unclear [21- 23]. For these reasons, screening of novel lectins from different sources is always desirable in order to utilize their beneficial properties in the field of biotechnology and biomedicine.
Although there is an abundance of information related to the major phytochemical components of P. amboinicus leaves is available, to the best of our knowledge, there is no information available regarding purification and characterization of lectin protein. With this background, the present investigation focused on the purification of the lectin from P. amboinicus leaves and the evaluation of its antioxidant functional attributes, which may have potential bio-therapeutic properties. Here, we report the purification and characterization of the physico- chemical properties of lectin from Indian borage leaves. The biological activity of the purified lectin was examined for its biotechnological applications.
Materials and Methods
Chemicals and reagents
Chemicals, reagents, and solvents obtained from Himedia (India), SRL (India) and SD Fine (India), which were of analytical grades.
Preparation of crude protein extract from Indian borage leaves
Plant materials were collected from Bio-Park Sector 2 at the Bangalore University campus, Bengaluru. The collected leaves were washed thoroughly with tap water to remove dust from the leaf surface. Fresh plant material (10 g) was homogenized with 20 mL of 0.025 M phosphate buffered saline (PBS) pH 7.2 using a pestle and mortar and then stored at 4°C for overnight. The plant extracts were centrifuged at 7000 rpm for 15 min at 4°C. The supernatant was collected and stored at 4°C for further analysis.
Determination of haemagglutination activity assay
The presence of lectins in plant extracts was examined by determining the haemagglutination activity
Preparation of native erythrocytes sample: A chicken blood sample was obtained by venipuncture of healthy animals from local butcher shops in Bengaluru. One milliliter of blood sample was centrifuged at 7000 rpm for 15 min at room temperature to separate erythrocytes from the serum, which were then washed thrice with 0.01 M PBS (pH 7.2 containing 0.15 M NaCl). Red blood cells (RBCs) concentrate obtained were diluted with PBS to make a 2% (v/v) blood suspension and stored at 4ºC until further use [24].
Haemagglutination activity assay: The haemagglutination activity assay was performed in a 96-well microtitre V-plate using a 2% suspension (v/v) of native chicken erythrocytes [24]. In this step, two-fold serial dilution of 50 μL of crude protein extract with 50 μL of 0.01M PBS (pH 7.2) was made in a microtitre plate. Then, 50 μL of 2% (v/v) suspension of chicken erythrocytes was added to each well and incubated at room temperature for 30 min. PBS without plant extract was used as a control. Haemagglutination activity was visually observed when more than 50% of the RBCs in the well were agglutinated, which depicted as mat formation, and it was tested as positive. Negative haemagglutination activity showed button formation at the bottom of the well. Haemagglutination activity was expressed as haemagglutination titre units (HU). The haemagglutination titre is defined as the reciprocal of the highest dilution displaying positive haemagglutination activity [25].
Haemagglutination inhibition test
The haemagglutination inhibition test was performed to investigate the carbohydrate-binding specificity of the screened lectin using different carbohydrates as mentioned in our previous article [26].
Purification of lectin from Plectranthus amboinicus
Protein precipitation: The ammonium sulphate precipitation method was applied to fractionate the proteins from the crude aqueous extract using different salt concentrations i.e., 0-20, 20-40, 40-60, 60-70, and 70-80 (w/v). The protein precipitate of each concentration was dissolved in 0.025 M PBS (pH 7.2) and dialyzed with the same buffer at 4°C for 18 h as mentioned in Roopashri and Savitha [27].
Preparation of affinity chromatography column using Seralose-4B matrix: An affinity chromatography column was prepared using Seralose-4B beads (SRL, India) as a matrix, which was coupled with dextrose (Himedia, India) sugar using epichlorohydrin as a cross-linking agent. As the tested lectin protein showed a higher specificity for dextrose, it was possible to retain PAL in a one-step purification approach. Column preparation was done according to Adenike [28] method with a slight modification. Fifty milliliter of Seralose- 4B beads were washed with 500 mL of MQ water and resuspended in 100 mL of 0.2 M carbonate buffer (pH 11) containing 1 mL of epichlorohydrin, then matrix was kept in a shaking incubator for 2 h at room temperature later washed with MQ water. The activated beads were equilibrated in 0.2 M carbonate buffer containing dextrose (0.2 M) and shaken at room temperature for 24 h followed by washing with MQ water as well as carbonate buffer and then resuspended in glycine solution (100 mg in 10 mL of 0.2 M carbonate buffer, pH 11). The suspension was shaken again for 2 h at room temperature and washed with carbonate buffer. A homogenized Seralose 4B-dextrose slurry (10 mL) was transferred into a column (1.5 x 30 cm) and equilibrated with 0.025 M PBS at pH 7.2 containing 0.15 M NaCl until the absorbance of elutes read <0.05 at 280 nm. This ensured the absence of any other impurities in the matrix.
Purification of Plectranthus amboinicus lectin: The dialyzed protein precipitate containing the soluble lectin protein was purified by affinity chromatography. The sample was loaded onto a cross-linked Seralose 4B-dextrose matrix column (1.5 x 30 cm) and washed with PBS at a flow rate of 1 mL min-1 until the column effluent had an absorbance of <0.05 at 280 nm. An elution buffer such as 0.2 M dextrose in 0.025 M PBS (pH 7.2) was used to elute the adsorbed lectin proteins by collecting a fraction of 1 mL min-1 and measured the protein content at 280 nm as well as the lectin content by HA assay using 2% chicken erythrocytes. Finally, the active fractions were pooled, dialyzed, lyophilized and stored at -20°C until use [28].
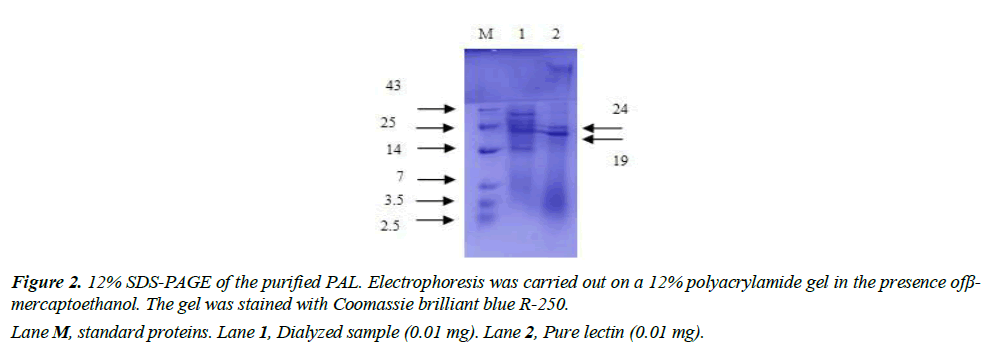
Homogeneity and molecular mass determination: The degree of protein purity and the molecular weight were assessed by SDS-PAGE. The protein sample was mixed with sample buffer containing 12% SDS and 6% β-mercaptoethanol and heated at 90°C for 5 min. The standard Coomassie Brilliant Blue method was applied to stain the gel [29].
Protein and carbohydrate determination: The total protein content was determined by Bradford method [30].
Characterization of purified PAL
Effect of pH on lectin activity of Plectranthus amboinicus: The effect of pH on HA of PAL was examined using various 0.1 M buffers such as citrate phosphate (pH 4.0–6.0), Tris- HCl (pH 6.0–9.0) and glycine-NaOH (pH 9.0–11.0), followed by incubation of the protein samples at 28°C for 60 min. The samples were then tested for residual hemagglutination activity. The hemagglutinating activity of the lectin sample maintained at pH 7.2 for 60 min was considered as the control and its activity was expressed as a percentage of relative activity [31].
Effect of temperature on lectin activity of Plectranthus amboinicus: The effect of temperature on purified lectin was tested in the range of 30-100°C for 30 min. The samples were immediately cooled in an ice-bath and residual haemagglutination activity was assayed. The sample incubated at 28°C for 30 min was considered as the control and its activity was expressed as a percentage of relative activity.
Effect of metal ions on lectin activity of Plectranthus amboinicus: The effect of metal ions on the haemagglutinating activity of purified PAL was studied. The protein sample was pre-incubated at 28°C for 60 min with 5 mM MgCl2 and CaCl2, followed by HA assay. Relative activity percentage was applied to express lectin activity.
Fourier Transform Infrared Spectroscopy (FTIR): For protein secondary structure analysis, a sample was prepared by grinding 1 mg of purified protein with 150 mg of spectroscopic grade KBr powder and compressing into a 1 mm pellet. The infrared spectrum of PAL was obtained with FTIR (Shimadzu, Kyoto, Japan) setting the wave number between 400-4000 cm−1. The obtained spectra were processed using Peakfit v4.12 software (SeaSolve, San Jose, CA, USA).
DPPH free radical scavenging assay: The ability of PAL to scavenge free radicals in a sample was examined by the in vitro 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay as described by Blois [32]. For this assay, 100μg/mL of the sample was dissolved in methanol and mixed with 2 mL of 0.1 mM DPPH solution, and then incubated in the dark for 20 min. The absorbance of the sample was measured at 517 nm in a UV-Vis spectrophotometer. The percentage of fee radical scavenging activity of PAL at different concentrations was estimated and compared with the ascorbic acid as standard. The DPPH solution alone was used as a control. The antioxidant activity was calculated as follows
% radical scavenging activity = 100 x (A0 - As)
A0
Where, A0 is absorbance of control and As is absorbance of sample.
Results and Discussion
Haemagglutination activity and haemagglutination- inhibition assays
In the present study, Indian borage (Plectranthus amboinicus) leaves were collected from Bio-park sector II at Bangalore University campus, Bengaluru, India. The plant extract was tested for haemagglutination activity with native erythrocytes of chicken to evaluate the presence of lectin and the quantity of lectin. The results showed that crude protein extracts from leaves exhibited the presence of lectin by agglutinating animal erythrocytes, which showed a higher haemagglutinating titer value i.e., 128 HU with 2% chicken native erythrocytes. Crude protein extract of Plectranthus amboinicus leaves was further examined for haemagglutination-inhibition test to determine their carbohydrate-binding specificity. For this, different carbohydrate such as mono- and oligosaccharides were used. The lectin of Indian borage plant exhibited carbohydrate- binding specificity for following carbohydrates such as D-galactose, dextrose, sucrose, and D-raffinose, which proved to be potent inhibitors of haemagglutinating activity of Indian borage lectin. Minimal concentrations (25, 50, and 200 mM) of the above-mentioned various sugars completely inhibited 4 haemagglutinating units (HU) of the Indian borage lectin. Dextrose was the most effective inhibitor of carbohydrates examined. Other carbohydrates, such as D-raffinose and sucrose showed only marginal inhibitory effects. Other carbohydrates that showed no haemagglutinating-inhibition activity included D-fructose, L-rhamnose, L-arabinose, lactose, D-mannose, D-xylose, D-ribose, stachyose, and D-cellobiose (Table 1). According to previous reports, the majority of plant lectins belonging to the Lamiaceae family showed weak affinity for simple sugars such as fructose, glucose, sucrose, etc. They were completely inhibited by glycoproteins such as albumin, azoalbumin, asialo bovine submaxillary mucin, calf asialofetuin, human serotransferrin, fetuin, N-acetylgalactosamine (GalNAc), desialylated mucins, and azocasein, indicating specificity for complex sugars. Therefore, these lectins have been categorized as complex carbohydrate-binding proteins and are efficiently used as ligands in affinity chromatography [33-36]. In this study, lectin from P. amboinicus showed greater affinity for simple sugars as well as for complex sugars.
| Sugars (400 mM) | Aqueous extract of Indian borage lectin (4 HU) |
|---|---|
| D(+) galactose | 50 |
| Dextrose | 25 |
| D(+) fructose | a- |
| D(+) lactose | a- |
| Maltose | a- |
| Sucrose | 200 |
| L(+) stachyose | a- |
| D(+) xylose | a- |
| D-ribose | a- |
| D(+) raffinose | 200 |
| L(+) arabinose | a- |
| D(+) mannose | a- |
| D-cellobiose | a- |
| L-rhamnose | a- |
Table 1. Haemagglutination-inhibition test of Indian borage agglutinin with sugars
A systematic survey of lectins was conducted on genera from the Lamiaceae family, including Hyptis suaveolens, Salvia, Moluccella, Glechoma hederacea, Lavandula, Lepechinia, Ocimum, Scutellaria, and Clerodendrum. They showed some common features at the level of their molecular characteristics. They appear as dimer, trimer or tetramer proteins and are more active at the neutral pH [37, 38]. However, the lectin from the Plectranthus plant has been little explored despite their great diversity. Therefore, in this study Plectranthus amboinicus was selected, given its prevalence in tropical regions in India, as well as its availability in significant quantities. To the best of our knowledge, this study is the first one to report the presence of a lectin in P. amboinicus leaves. Many studies have reported the detection and characterization of major phytochemical compounds in P. amboinicus, simultaneously proving their beneficial attributes by in vitro as well as in vivo approaches. Welton [39] were the first to report the presence of lectins in Plectranthus barbatus leaves. This lectin agglutinated rabbit erythrocytes and showed a greater affinity for the sugars such as ribose and galactose. Most of the Lamiaceae family lectins agglutinate erythrocytes of human blood type B+ve, rabbit, rooster, sheep, and cow. However, in the present study, P. amboinicus leaf lectin exhibited higher haemagglutination activity towards chicken erythrocytes.
Purification of lectin from P. amboinicus
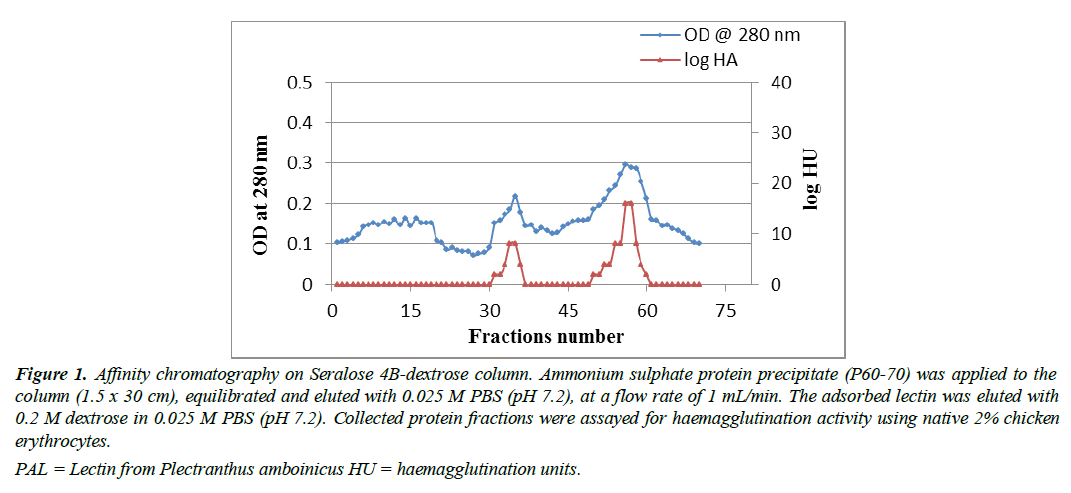
Protein purification was performed using affinity chromatography (AC). Haemagglutinin activity was found in the fraction which was precipitated at a salt concentration of P60–70. While HA was not present in fractions such as P0-20, 20-40, 40-60 and 70-80. Purification by affinitychromatography was then performed using P60-70 protein precipitate. Figure 1 represents the P60-70 affinity chromatography on a Seralose 4B-dextrose slurry column which showed lectin activity in 31 to 36 fractions and 50 to 56 fractions, whereas fractions 57, 58, and 60 showed higher haemagglutination activity. The elution buffer of 0.2 M dextrose used in this study was able to recover more protein, thus attained a purification fold of 25 with a specific activity increase of 427 HAU per mg as summarized in Table 2. Therefore, from 2.4 mg of total protein in AE, was able to purify 0.3 mg of protein by AC, which had a specific HA of 427 titre mg-1 protein, resulting in a 67% yield. The lectin purity was assessed by SDS-PAGE in the reduced form, where two distinct bands were observed. This analysis revealed that PAL was fragmented into two subunits with molecular weight of 19 kDa and 24 kDa (Figure 2). The haemagglutination activity in 96 well microtiter plate (Figure 3), as well as the titer value of the aqueous extract, dialyzed protein fraction (0/70), and purified lectin are summarized in Table 3. A similar lower molecular weight of lectin was reported in Plectranthus barbatus leaves, which showed affinity for rabbit erythrocytes. Its specific hemagglutinating activity was observed to be 177.78 and it had less activity for monosaccharides such as fructose, N-acetylglucosamine, glucose, and mannose, but no activity for ribose and galactose. These data indicated that the lectins of Plectranthus barbatus leaf extract were more specific to ribose/galactose [39].
| Sample | Volume (mL)a | HA (HU/mL) b | Total activity(axb) | Total Protein (mg/mL)c | Specific activity (b/c) | Recovery Yield (%) d | Purification (fold) |
|---|---|---|---|---|---|---|---|
| Aqueous extract (AE) | 15 | 16 | 240 | 2.4 | 7 | 100 | 1 |
| P60-70 | 6 | 32 | 192 | 1.85 | 17.29 | 80 | 2.47 |
| Affinity column (AC) | 1 | 128 | 128 | 0.3 | 427 | 67 | 25 |
a Total volume of the sample taken.
b Haemagglutination activity of lectin.
c Total protein content in the sample.
b/c Specific haemagglutination activity was calculated by the ratio of HA and protein content (titre mg-1 ).
d Yield corresponded to the amount of protein in AE recovered after each purification stage.
HU = haemagglutination units.
Table 2. Summary of Plectranthus amboinicus lectin purification
| 2 % Chicken erythrocytes | HU mL-1 | ||
|---|---|---|---|
| Crude extract | Dialyzed sample | Pure lectin | |
| Native cells | 16 | 32 | 128 |
Table 3. Titration values exhibited by lectin of Plectranthus amboinicus with chicken erythrocytes
Figure 1: Affinity chromatography on Seralose 4B-dextrose column. Ammonium sulphate protein precipitate (P60-70) was applied to the column (1.5 x 30 cm), equilibrated and eluted with 0.025 M PBS (pH 7.2), at a flow rate of 1 mL/min. The adsorbed lectin was eluted with 0.2 M dextrose in 0.025 M PBS (pH 7.2). Collected protein fractions were assayed for haemagglutination activity using native 2% chicken erythrocytes.
PAL = Lectin from Plectranthus amboinicus HU = haemagglutination units.
According to the literature data, most of the Lamiaceae lectins were precipitated at higher concentrations of ammonium sulphate (60-80%). Lectin purification from the seeds of Ocimum basilicum (OBSL) belongs to one of the genera of the Lamiaceae family, which showed the presence of a trimer protein with a molecular weight of 163 kDa. The lectin was purified using fetuin-agarose affinity resin, which showed a higher affinity for erythrocytes of human blood group B+ve, rabbit, cow and sheep. Another genus in the Lamiaceae family, belonging to Scutellaria baicalensis, showed the presence of a lectin in the seeds, and its molecular weight was 62.23 kDa that means it consists of only one polypeptide chain without intra-chain disulfide bonds. The molecular weight of the Clerodendrum infortunatum lectin was 120 kDa as a homotetramer, whereas MALDI-TOF analysis showed four identical subunits of 30 kDa [34]. The Moluccella laevis lectin had a unique structural arrangement with 67, 42 and 26 kDa subunits in the native gel, whereas in the presence of a reducing agent (8 M urea) it showed a 26 kDa subunit that was active only after affinity chromatography [40]. The lectin from Glechoma hederacea showed two polypeptide bands of 28 and 26 kDa on reduced SDS-PAGE. Many previous reports on Lamiaceae lectins revealed that they contain more than one subunit with different molecular weights.
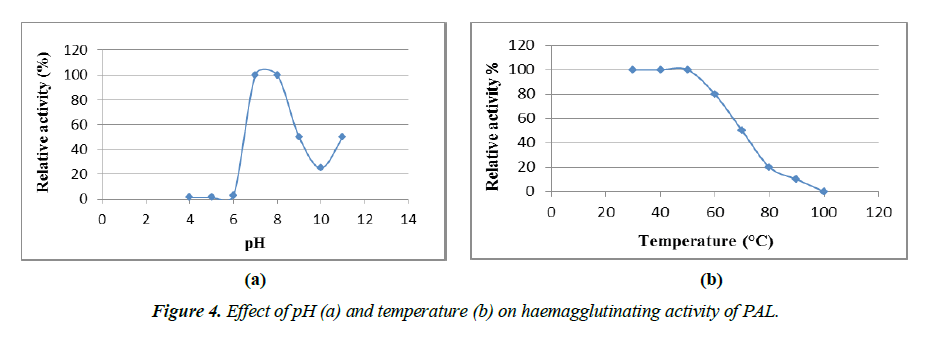
Effect of pH and thermal stability on PAL
In addition, the purified lectin was also tested for Haemagglutinating Activity (HA) at different pH, temperature, and divalent metal ions. PAL exhibited HA over a broad range of pH from 6.5 to 11 (Figure 4a). The optimum pH for PAL activity was 7-7.5, below which activity was moderately low. At pH 8.0, the PAL was sensitive and lost almost 85% of HA. However, HA again increased at pH 11.0 as the optimum pH. Similarly, in the case of Ocimum basilicum seeds, the lectin showed optimal activity at pH 7.5, after which the HA slowly declined. But, as the pH increased from 11 to 12, its activity began to increase again, providing another optimal pH range for OBSL. A purified lectin from a similar genus, i.e. from the O. sanctum leaves, was constant in the pH range of 5 to 12 [41, 42]. However, lectin activity with such extreme pH stability is not occasional, similar results have been shown with Glechoma hederacea which was stable in the pH range of 3-12, fog egg stability up to pH 10 and Phaseolus vulgaris ranging from 1 to 14 [43-45].
The thermal stability of PAL was examined in the range of 30-100°C. Its hemagglutinating activity was stable up to 50°C for up to 30 min, but 50-85% of HA was reduced by heating at 70°C to 90°C for up to 30 min. Complete inactivation of HA occurred at 100°C after 30 min (Figure 4b), indicating that PAL had intermediate thermo-stability. When the PAL aliquot was preserved at 4°C for up to 30 days, there was no apparent loss of lectin activity, confirming its high stability. Lectins with high thermal stability are general characteristics of most plants belonging to the Lamiaceae family. Purified lectin from Ocimum sanctum retained HA at 80°C for 30 min [40]. Similarly, the Glechoma hederacea lectin resisted temperature up to 65°C [37]. Purified lectins from species of Gleheda and Salvia bogotensis retained 50% of their activity up to 60°C [46]. However, lectins from a few genera of the Lamiaceae family lose their activity at lower temperatures. Species such as Salvia sclarea and Lepechinia bullata exhibited poor activity at lower temperatures, i.e. above 37°C, and were completely inactivated at 60°C [46]. Most of the Lamiaceae lectins showed activity at optimal pH (7 and 8), whereas 50% of the activity was reduced at 50-70ºC, these results were almost consistent with the present report. The haemagglutinating activity of PAL was not inhibited by divalent metal ions such as Ca2+ and Mg2+ compared to the initial 4 HU, displaying its strong stability.
FTIR analysis
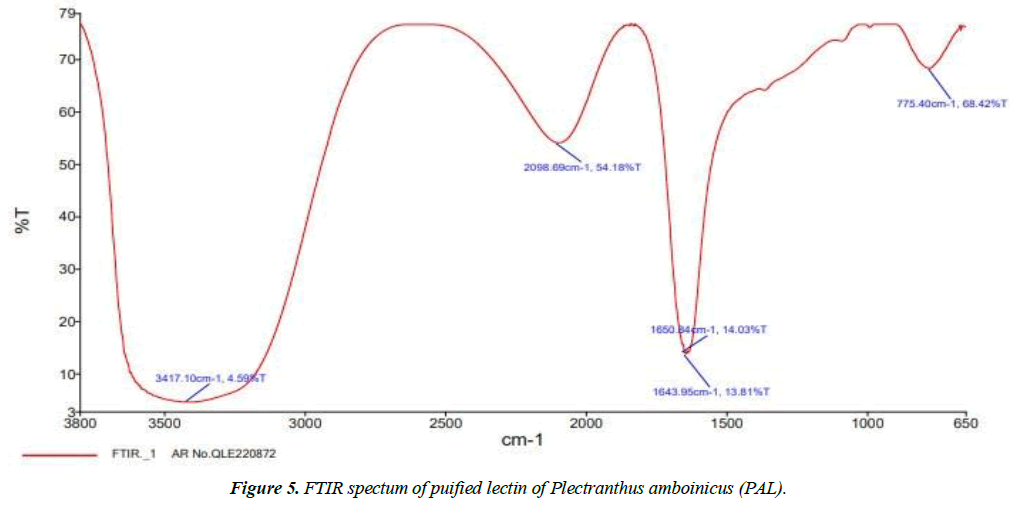
The FTIR method was used to categorize the different functional groups present in the purified PAL responsible for the biological activity. This was performed using a spectrophotometer to identify characteristic peak values and their functional groups.
The band intensities in different regions of the PAL spectra were analyzed and depicted in Figure 5. The shift in the peak at 3417 cm-1 was a protein absorption band characteristic of the overlapping of O-H and N-H stretching vibrations, indicating the presence of intramolecular H-bonds, intermolecular H-bonds, and amidogens in the PAL. Similar results were obtained for the secondary structure of the lectin from the Loach skin mucus, a kind of nutritious freshwater fish, showing a strong and broad absorption band at 3388.75 cm-1 [47]. Another peak at 2098 cm-1 resembles multiple bonding of the nitrile compound. In addiion, peak shifts from 1650 cm-1 to 1643 cm-1 indicated a possible contribution of C=O stretching of the amide compound I, i.e., band vibrations in the keto group. The absorption of the alpha-helix, quantified as 14.03% at 1650 cm-1 and the disordered absorption of 13.81%, which appeared at 1643 cm-1 are presented in Table 4. The peak shift at 775 cm-1 suggested the involvement of the C-Cl stretching vibration of aliphatic chloro compounds [48,49]. This study revealed the presence of amide I, carboxylic acid, ketones, primary amines, and aliphatic chloro compounds obtained as major peaks. These stretching vibrations suggest that groups of them may be involved in biological activity.
| List of Peak Area/Height | ||
|---|---|---|
| Peak Number | X(cm-1) | Y(%T) |
| 1 | 3417.1 | 4.59 |
| 2 | 2098.69 | 54.18 |
| 3 | 1650.84 | 14.03 |
| 4 | 1643.95 | 13.81 |
| 5 | 775.4 | 68.42 |
Table 4. FTIR spectral peak values
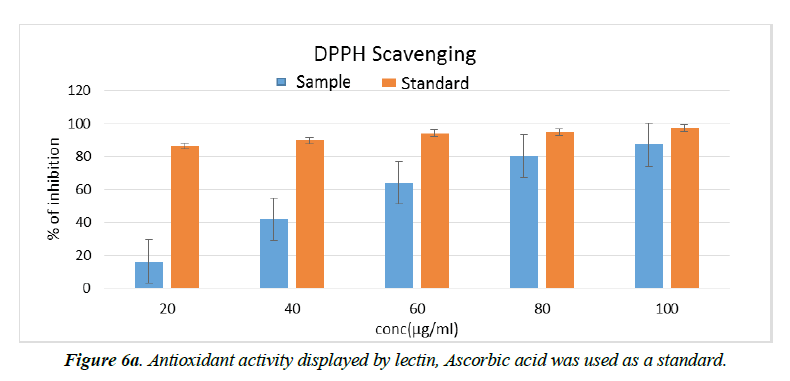
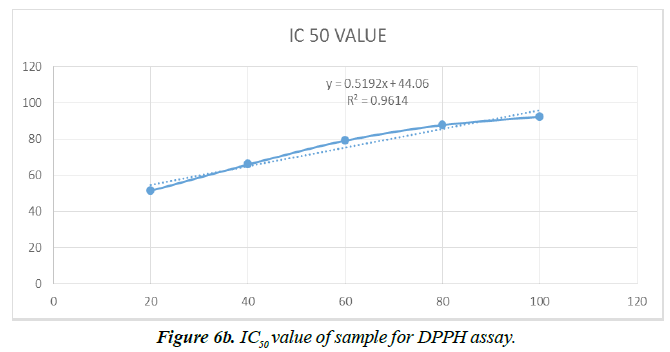
Antioxidant activity
However, information regarding the antioxidant activity of lectin from Plectranthus amboinicus leaves is not available and requires careful research in order to obtain a potential bio-therapeutic, safer agent. Therefore, experimental study was performed to confirm the presence of antioxidant activity of purified lectin from P. amboinicus leaves. The moderate antioxidant potential of PAL was guaranteed based on the values obtained in selected in vitro assay. The antioxidant activity of PAL at different concentrations is shown in Figure 6a. Purified PAL suspended in methanol exhibited a higher (P ≤ 0.05) free radical scavenging activity of DPPH at 100 μg/mL (∼87%) whereas activity was low at other concentrations. The antioxidant standard, i.e. ascorbic acid, used in this assay exhibited the same activity at 40 μg/mL. Hence, PAL revealed antioxidant activity with an IC50 value of 11.44 ± 0.1 mg mL-1 (Figure 6b). On the other hand, the lectin of Clerodendrum infortunatum (Family-Lamiaceae), which exhibited a higher antioxidant activity with IC50 value of 6.1 ± 0.1 mg mL-1 compared to PAL [32]. This is the first report on the purification and characterization of a lectin from Plectranthus amboinicus leaves. However, Welton [39] reported other Plectranthus species, i.e. Plectranthus barbatus, on the presence of ribose/galactose-binding lectins in saline extract (SE), leaf decoctions and infusions. They also reported that Plectranthus barbatus lectin activity was not affected by the larval proteases that were resistant to hydrolysis by intestinal proteases of third instar A. aegypti larvae (L3).
Conclusion
Based on this study, it can be concluded that the Plectranthus amboinicus lectin shows a higher haemagglutinating titer with significant carbohydrate binding specificity. Interesting results obtained with respect to lectin, i.e. high pH and thermal stability and moderate purification yield, as well as an antioxidant rich property, may lead to further structural and molecular studies that would pave the way to explore the use of lectin for therapeutic purposes.
Conflict of interest
"The authors declare no potential conflicts of interest."
Statements and Declarations
Data available on request from the authors.
Acknowledgements
The authors wish to gratefully acknowledge University Grants Commission (UGC)-Dr. D.S. Kothari Postdoctoral fellowship scheme (No.F.4-2/2006(BSR)/BL/18-19/0159), India, for providing financial support to carry out this research work and Bangalore University for allowing us to utilize the laboratory facilities at the Department of Microbiology, Biotechnology and Food Technology, Bengaluru, India.
References
- Paton A, Mwyanyambo M, Govaerts RH, et al. Nomenclatural changes in Coleus and Plectranthus (Lamiaceae): a tale of more than two genera. PhytoKeys. 2019;129:1-58.
- Khare RS, Banerjee S, Kundu K (2011) Coleus aromaticus benth - a nutritive medicinal plant of potential therapeutic value. Int J Pharma Bio Sci. 2011;2(3).
- Annapurani S, Priya R (1999) Antimutagenic, antitumorogenic, and antigenotoxic effects of polyphenol extracts of selected medicinal plants. Indian J Nutr Diet 1999:431-35.
- Dostalova Z, Calvete JJ, Sanz L. Topfer PE (1995) Boar spermadhesin during mouse embryogenesis. J Cell Biochem 36:1-14.
- Kasai KI, Hirabayashi J. Galectins: a family of animal lectins that decipher glycocodes. J Biochem. 1996 ;119(1):1-8.
- Gabius HJ. Animal lectins. Eur J of Biochem. 1997 Feb;243(3):543-76..
- Beisel HG, Kawabata SI, Iwanaga S, et al. Tachylectin-2: crystal structure of a specific GlcNAc/GalNAc-binding lectin involved in the innate immunity host defense of the Japanese horseshoe crab Tachypleus tridentatus. EMBO J. 1999;18(9):2313-22.
- Costa RB, Campana PT, Chambergo FS, et al. Purification and characterization of a lectin with refolding ability from Genipa americana bark. Int J Biol Macromol. 2018;119:517-23.
- Carvalho AS, da Silva MV, Gomes FS, et al. Purification, characterization and antibacterial potential of a lectin isolated from Apuleia leiocarpa seeds. Int J Biol Macromol 2015;75:402-8.
- Wilches TA, Rojas-Caraballo J, Sanabria E, et al. Purification and biochemical characterization of a t/tn specific lectin from Lepechinia bullata seeds (Lamiaceae). Int J Pharm Pharm Sci 9:165-74.
- Sharma M, Vishwanathreddy H, Sindhura BR, et al. Purification, characterization and biological significance of mannose binding lectin from Dioscorea bulbifera bulbils. Int J Biol Macromol 2017;102:1146-55.
- Bobbili KB, Pohlentz G, Narahari A, et al. Coccinia indica agglutinin, a 17 kDa PP2 like phloem lectin: affinity purification, primary structure and formation of self-assembled filaments. Int J Biol Macromol 2018;108:1227-36.
- Thakur K, Kaur T, Singh J, et al. Sauromatum guttatum lectin: Spectral studies, lectin-carbohydrate interaction, molecular cloning and in silico analysis. Int J Biol Macromol 2017;104: 1267-79.
- Ye XY, Ng TB, Tsang PWK, et al. Isolation of a homodimeric lectin with antifungal and antiviral activities from red kidney bean (Phaseolus vulgaris) seeds. J Protein Chem 2001;20:367-75.
- Chan YS, Wong JH, Ng TB. A glucuronic acid binding leguminous lectin with mitogenic activity toward mouse splenocytes. Protein Pept Lett 2011;18:194–202.
- Díaz CL, Melchers LS, Hooykaas PJ, et al. Root lectin as a determinant of host–plant specificity in the Rhizobium–legume symbiosis. Nature. 1989;338(6216):579-81.
- Chu CY, Liao WR, Huang R, et al. Haemagglutinating and antibiotic activities of fresh water microalgae. World J Microbiol Biotechnol 2004;20:817-25.
- Hakomori SI. Glycosphingolipids as differentiation-dependent, tumor-associated markers and as regulators of cell proliferation. Trends Biochem Sci. 1984;9(10):453-9.
- Fukuda M. Cell surface glycoconjugates as on codifferentiation markers in hematopoietic cells. Biochemica et Biophysica Acta 1984;780:119-150.
- Muramatsu T. Developmentally regulated expression of cell surface carbohydrates during mouse embryogenesis. J Cell Biochem 1988;36:1-14.
- Rogers DJ, Swain L, Carpenter BG, et al. Binding of N-acetyl--D-galactosamine by lectins from species of the green marine algal genus, Codium. Clin Biochem. 1994;10:162-5.
- Lis H, Sharon N. Lectins: carbohydrate-specific proteins that mediate cellular recognition. Chem Reviews. 1998;98(2):637-74.
- Romulo FC, Jhonatas TV, Renato CFT, et al. A new mucin-binding lectin from the marine sponge Aplysina fulva (AFL) exhibits antibiofilm effects. Arch Biochem Biophys 2019;662:169-76.
- Hori K, Miyasawa K, Ito K. Some common properties of lectins from marine algae. Hydrobiologia 1990;204:561-66.
- Kotone S, Haruko O. Hemagglutination (Inhibition) Assay. Methods Mol Biol. 2014;1200:47-52.
- Roopashri AN, Savitha J. Screening of freshwater microalgae species for occurrence of lectins and their carbohydrate-binding specificity. J Appl Boil Sci 2022;16:24-34.
- Roopashri AN, Divyashree MS, Savitha J. High-sensitivity profiling of glycoproteins from ovarian cancer sera using lectin-affinity and LC-ESI-Q-TOF-MS/MS. Curr Res Biotech 2023;5:100122.
- Kuku A, Togun RA, Obuotor EM, et al. Acute toxicity and histopathology study of a galactose-specific lectin from the seeds of Tetracarpidium conophorum (African walnut)(Hutch and Dalz). Toxicol Environ Chem. 2012;94(3):583-92.
- Laemmli UK. Cleavage of structural proteins during the assembly of bacteriophage T4. Nature 1970;227:680-5.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248-54.
- Wang H, Gao J, Ng TB. A new lectin with highly potent antihepatoma and antisarcoma activities from the oyster mushroom Pleurotus ostreatus. Biochem Biophys Res Commun. 2000;275(3):810-6.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199-200.
- Benevides RG, Ganne G, da Conceição Simões R, et al. A lectin from Platypodium elegans with unusual specificity and affinity for asymmetric complex N-glycans. J Biol Chem. 2012;287(31):26352-64.
- Surya S, Haridas M. A New Galactose-Specific Lectin from Clerodendrum infortunatum. Iranian J Biotech. 2018;16(4).
- Wang H, Gao G, Ke L, et al. Isolation and Characterization of a Lectin-like Protein (SBLP) from the Dried Roots of Scutellaria baicalensis (Lamiaceae). Nat Prod Commun. 2018;13(12):1934578X1801301232.
- Dafalla MB, Ebrahim RM, Abdulaziz NM, et al. Isolation, Purification and Characterization of a Lectin from Ocimum basilicum Seeds (OBSL) with a Complex Sugar Specificity.
- Wang W, Peumans WJ, Rougé P, et al. Leaves of the Lamiaceae species Glechoma hederacea (ground ivy) contain a lectin that is structurally and evolutionary related to the legume lectins. Plant J. 2003;33(2):293-304.
- Turgut AC, Emen FM, Canbay HS, et al. Chemical characterization of Lavandula angustifolia Mill which is a phytocosmetic species and investigation of its antimicrobial effect in cosmetic products. J Turkish Chem Soc. 2017;4(1):283-98.
- de Almeida WA, Nova IC, da Silva Nascimento J, et al. Effects of Plectranthus barbatus leaf extract on survival, digestive proteases, midgut morphophysiology and gut microbiota homeostasis of Aedes aegypti larvae. South Afr J Bot. 2021;141:116-25.
- Alperin DM, Latter H, Lis H, et al. Isolation, by affinity chromatography and gel filtration in 8 M-urea, of an active subunit from the anti-(blood-group A+ N)-specific lectin of Moluccella laevis. Biochem J. 1992;285(1):1-4.
- Vemuri PK, Talluri B, Sharma A, et al. Isolation and characterization of a lactose-binding lectin from Ocimum sanctum. J Appl Pharm Sci. 2015;5(10):113-7.
- Okabe Y, Katayama N, Iwama M, et al. Comparative base specificity, stability, and lectin activity of two lectins from eggs of Rana catesbeiana and R. japonica and liver ribonuclease from R. catesbeiana. J Biochem. 1991;109(5):786-90.
- Sharma A, Ng TB, Wong JH, et al. Purification and characterization of a lectin from Phaseolus vulgaris cv.(Anasazi beans). J Biomed Biotechnol. 2009;2009.
- Vega N, Pérez G. Isolation and characterisation of a Salvia bogotensis seed lectin specific for the Tn antigen. Phytochem. 2006;67(4):347-55..
- Piller V, Piller F, Cartron JP. Isolation and characterization of an N-acetylgalactosamine specific lectin from Salvia sclarea seeds. J Biol Chem. 1986;261(30):14069-75.
- Sun PP, Ren YY, Zheng J, et al. Purification and characterization of a new lectin from loach skin mucus. J Chem. 2019;2019.
- Sahayaraja PA, Gowri J, Dharmalingama V, Shobanaa R, Prema AA ( 2015) Phytochemical screening by FTIR spectroscopic analysis of leaf and stem Extracts of Wedelia biflora. Int J Nano Corr Sci Engg 2:322-334.
- Singh RS, Walia AK. Lectins from red algae and their biomedical potential. J Appl Phycol. 2018;30:1833-58.
- Pohleven J, Štrukelj B, Kos J. Affinity chromatography of lectins. Affinity Chromatography. InTech. 2012:49-74.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref