Research Article - International Journal of Pure and Applied Zoology (2017) Volume 5, Issue 3
POLYGYNOUS MATING SYSTEM AND FIELD EVIDENCE FOR BIDIRECTIONAL SEX CHANGE IN THE GOBIID FISH TRIMMA GRAMMISTES
- *Corresponding Author:
- Kazuya Fukuda
Laboratory of Fish Behavioral Ecology
University of Marine Science and Technology, Japan
E-mail: kazuya.f47@gmail.com
Received 20th July 2017; Accepted 15th August 2017; Published 18th August 2017
Abstract
The mating system of the gobiid fish Trimma grammistes as well as bidirectional sex change in the species was investigated in Ito Beach, Chiba, Japan. Sixty-seven individuals appeared in the study area. The sex ratio in T. grammistes was femalebiased (22:45), and there was no significant difference between the total length of males and females. They formed social groups that consisted of one or two males and some smaller females. The largest individual in a group was always male. All aggressive behaviors were performed by the largest individuals in each social group regardless of their sex. The largest males monopolized all mating opportunities. These results indicated that the mating system of T. grammistes was harem polygyny. In some groups, however, small males existed. The reproductive status of small males (regardless of whether the small male had reproductive ability and opportunity) remained unclear. Two and three instances of female-to-male and male-to-female sex change were observed during the study period, respectively. In addition, we confirmed that one individual changed sex from female to male and vice versa. Most of these sex changes were observed when the social status had fluctuated; e.g., a large male had disappeared or newly joined the group. Therefore, the ability to undergo sex change in this species might evolve to maximize reproductive success in the harem polygyny, and corresponds well with the prediction of the size-advantage model.
Keywords
Mating system; Polygyny; Bidirectional sex change; Field observation; Gobiidae; Trimma grammistes
Introduction
Sequential hermaphroditism or sex change is widely known in teleosts (Munday et al., 2006; Sadovy de Mitcheson and Liu, 2008). Among these fishes, protogynous (female- -to-male) and protandrous (male-to-female) sex changes have been reported or suggested in at least 18 and eight families, respectively (Sadovy de Mitcheson and Liu, 2008; Shitamitsu and Sunobe, 2016). The size-advantage (SA) model successfully explains the adaptive significance and the evolution of sex change (Ghiselin, 1969; Warner, 1975, 1984). This model theoretically predicts that protogynous sex change should be favored in polygynous species. In these species, large (or aged) males obtain higher reproductive success than small (or young) males and similar-sized females resulting from female mate choice for larger males or male– male competition for female. Especially, as the large male monopolize reproductive opportunity for the above reason; the small male should suffer a serious loss of the reproductive opportunity. Therefore, these species reproduce as female when they are small, and change their sex from female to male when they become large. By contrast, the evolution of protandrous sex change is predicted in random mating (males mate with females randomly) (Warner, 1975,1984). Although the fecundity of female increases with their body size, males are reproductively successful regardless of their body size (or age) under this condition. Therefore, the male changes their sex to female when they become large. The SA model emphasizes that the evolution of sex change relates to the mating system. It has been supported by many empirical studies (Kuwamura and Nakashima, 1998; Munday et al., 2006; Avise, 2011).
In addition to these two types of sex change, bidirectional sex change has been reported in 31 species from seven families (Munday et al., 2010; Manabe et al., 2013; Kuwamura et al., 2015, 2016). Whereas most of these cases have been confirmed in aquaria, bidirectional sex change in the field has been reported in 11 species (Kuwamura and Nakashima, 1998; Munday et al., 2010; Manabe et al., 2013; Kuwamura et al., 2015, 2016). These bidirectional sex changers have polygynous or monogamous mating systems (Munday et al., 2010). In the polygynous species, the largest female changes sex to male after the disappearance of the dominant male. By contrast, sex change from male to female occurs when a male– male pair is established under a low density condition (Kadota et al., 2012; Kuwamura et al., 2014, 2015, 2016), or when two males exist in the same harem (Manabe et al., 2007a). However, the monogamous coral-dwelling gobiid fishes Paragobiodon echinocephalus and Gobiodon histrio change sex from male to female or female to male after establishing a pair of the same sex. It should be more advantageous to mate with a nearby consexual individual than a distant heterosexual one, because predation risk may increase as a result of moving between the habitat corals (Kuwamura et al., 1994; Nakashima et al. 1995; Munday, 2002).
Gobiidae is one of the families in which bidirectional sex change was detected (e.g. Munday et al., 2010; Sunobe et al., 2017). In particular, the sexuality has been well documented by field and aquarium observations and/or histological investigations in the genus Trimma (Sunobe and Nakazono, 1990; Sunobe and Nakazono, 1993; Shiobara, 2000; Manabe et al., 2007a, 2008; Sakurai et al., 2009; Cole, 2010; Sunobe et al., 2017; Fukuda et al., 2017). Trimma are small (average 2 cm in standard length) and colorful gobiid fish found on rocky and coral reefs, and contain about 110 to nearly 200 species including undescribed species (Winterbottom et al., 2014; Suzuki et al., 2015). Although the ability of bidirectional sex change has been confirmed or predicted in 29 species of this genus (Sunobe et al., 2017), the mating system and occurrence of sex change under natural conditions is known in only Trimma okinawae. The mating system of this species is polygynous and exhibits bidirectional sex change by alteration of social dominance (Sunobe and Nakazono, 1990; Manabe et al., 2007a).
Trimma grammistes is a small goby that inhabits rocky shore areas. Histological study showed that T. grammistes matures at over 14.0 mm in standard length. Their breeding season lasts from May to October in Suruga Bay, Japan. It is known that the gonad is simultaneously composed of ovary and testis in both sexes (Shiobara, 2000). Although Shiobara (2000) reported the bidirectional sex change of T. grammistes by manipulative experiments in aquaria, there is no information about the mating system and sex change of T. grammistes in the field. In this study, we investigated the reproductive ecology and sex change of T. grammistes at Tateyama, Japan.
Materials and Method
Field observations
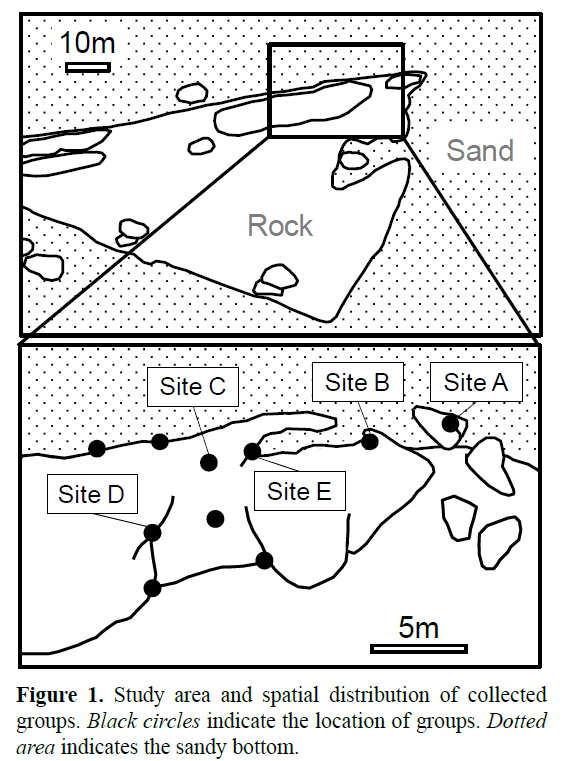
Underwater observation using scuba was conducted at Ito beach, Tateyama, Japan (34°95’ N; 139°77’ E) from June to August 2015. The study area was a rocky reef approximately 35 m length (Figure 1) that was situated 400 m from the shore at depths of 18-24 m. The water temperature ranged from 14.0 to 25.5° C during the study period.
T. grammistes inhabited the rocky caves or crevices. Thus, we collected individuals occurring in the study area by hand nets at the beginning of June 2015. Specimens were collected from 10 sites (Figure 1) and transported to the laboratory. All individuals were anesthetized with quinaldine and measured for total length (TL) to the nearest 0.5 mm and sexed by the shape of the urogenital papilla: long and tapered posterior in male, and bulbous with several processes at the papillae opening in female (Shiobara, 2000). All individuals were marked by subcutaneously injecting a visible implant Elastomer Tag (Northwest Marine Technology Inc., Shaw Island, WA, USA) for individual identification, and released at their collection sites the next day. After these procedures, we chose five sites (A, B, C, D and F) from the above 10 sites for continuous observation (Figure 1). Six individuals immigrated to the observation sites during the study period (see Results). These individuals were captured, anesthetized with quinaldine, measured for TL and sexed using a loupe under water. We also identified these individuals based on the lateral color pattern and some scars. After these procedures, these individuals were released at their collection site.
To reveal the mating system and social structure, we conducted continuous observation at five sites (A-E). T. okinawae, the species which is closely related to T. grammistes, spawns mainly in the morning (Sunobe and Nakazono, 1990). Thus, we observed each group for 5 min twice a day between 0900 and 1200. In each observation, we recorded the location of all individuals at the study sites by marking them on a scaled-down map of the study site. The home ranges of each individual were defined as the area within the most peripheral dots. We defined individuals whose home range overlapped and/or were located closely together within the same cave or hole for ≥ 3 days as a social group. If an individual immigrated to another social group without returning to their previous groups for 3 days, we regarded the individual as a member of the new social group. We counted the occurrence of social behaviors (i.e., aggressive behavior, courtship behavior and spawning) and recorded the individual identity of the participants involved in such social behavior.
To reveal the occurrence and adaptive significance of sex change in natural conditions, we sexed on the basis of the shape of the urogenital papilla when the individuals experienced a change in social condition according to the methods mentioned above.
Statistical analyses
We used Welch’s t-test, binomial test, chi-square test for statistical analysis of the difference of body size between sexes, the sex ratio at the beginning of the observation, the ratio of inter group movement in sex, respectively. To examine the effects of body size and sex on social group movement (including disappearance and immigration), we used a generalized linear model (GLM, implemented in R) with a binomial error distribution, because the response variable is binary (an individual moved or did not), and a logistic-link function. We included TL, sex and an interaction term between TL and sex as a fixed effect. All statistical calculations were conducted using R version 3.3.0.
Results
Size distribution of the sexes and sex ratio
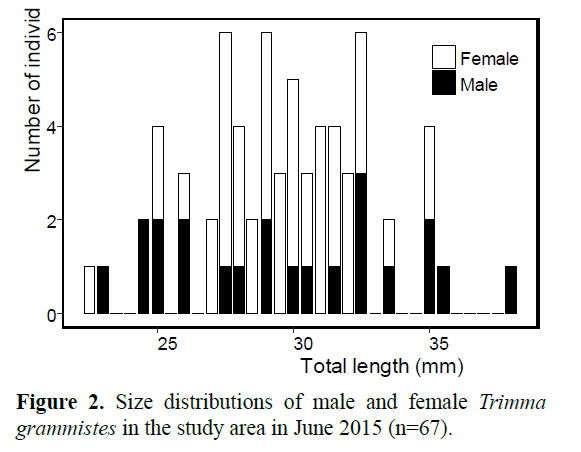
Of the 67 fish captured, 22 and 45 were identified as males (mean ± SD = 29.7 ± 4.3 mm TL, range =23.0–38.0 mm, N=22) and females (29.6 ± 2.6 mm TL, range =22.5-35.0 mm, N=45), respectively. There was no significant difference between the TL of males and females (Welch’s t-test, t=0.13, P>0.1) (Figure 2). The sex ratio in the study area was biased towards females (binomial test, P<0.01, n=67).
Social group and behavioral interaction
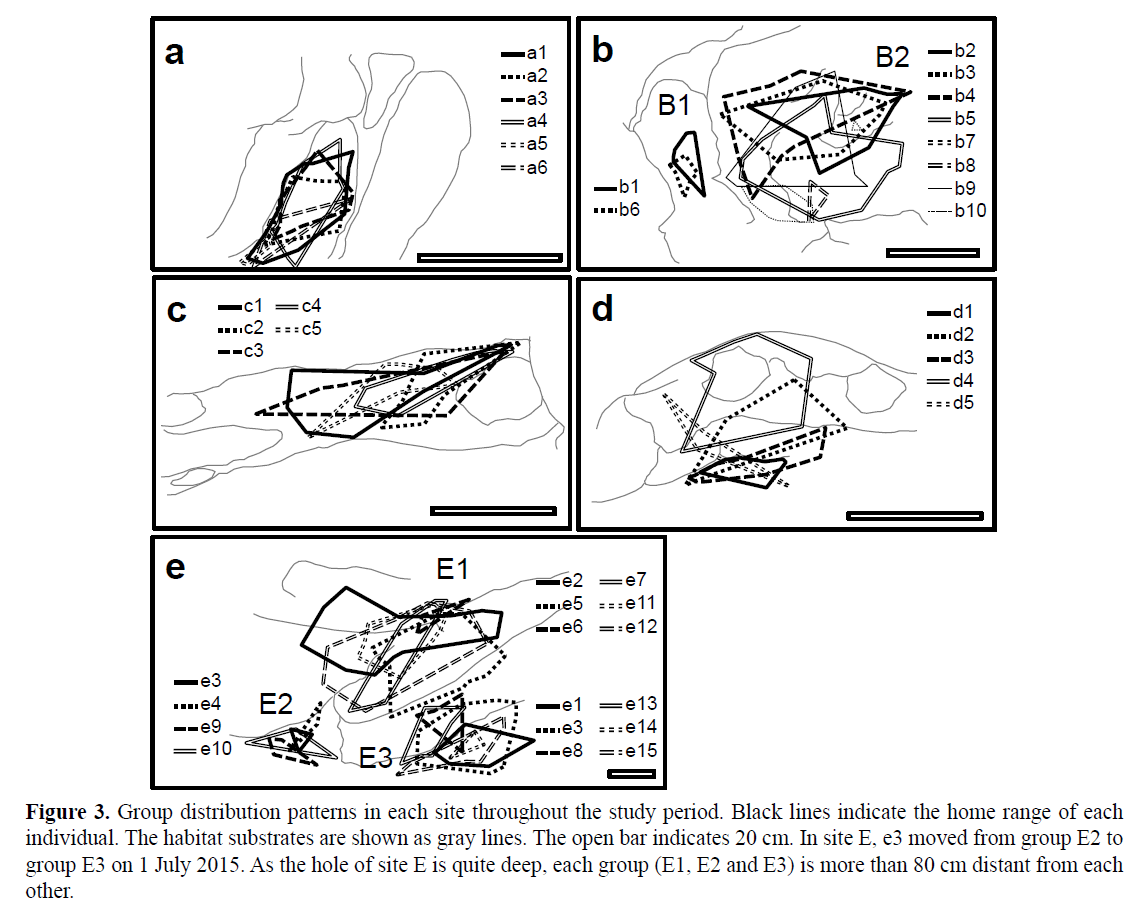
A total of 41 individuals were observed throughout the study period. Their size and sex at the beginning of observation are listed in Table 1. Eight social groups were found in five sites (A-E): group A (three individuals), group B1 (two individuals), group B2 (eight individuals), group C (five individuals), group D (five individuals), group E1 (six individuals), group E2 (four individuals) and group E3 (two individuals) at the beginning of observation (Figure 3). Group B1, D, E2 and E3 comprised one male and one to four females, while group A, B2, C and E1 comprised two males and two to six females (Figure 4). At the beginning of observation, the largest individual was male in 75% of eight social groups. However, the largest individual was male in all social groups at the end of observation.
| Location | ID | TL (mm) | Sex | Location | ID | TL (mm) | Sex |
|---|---|---|---|---|---|---|---|
| Site A | a1 | 32.5 | Female | Site D | d1 | 32.5 | Male |
| Site A | a2 | 31.5 | Male | Site D | d2 | 31.5 | Female |
| Site A | a3 | 28 | Female | Site D | d3 | 27.5 | Female |
| Site A | a4 | 26 | Male | Site D | d4 | 26 | Female |
| Site A | a5 | 23.5 | Female | Site D | d5 | 25 | Female |
| Site A | a6 | 23 | Female | Site E | e1 | 37 | Male |
| Site B | b1 | 35 | Male | Site E | e2 | 36 | Male |
| Site B | b2 | 32.5 | Male | Site E | e3 | 35 | Female |
| Site B | b3 | 32 | Female | Site E | e4 | 35 | Male |
| Site B | b4 | 32 | Female | Site E | e5 | 31.5 | Female |
| Site B | b5 | 31 | Female | Site E | e6 | 31.5 | Female |
| Site B | b6 | 29 | Female | Site E | e7 | 31 | Female |
| Site B | b7 | 28.5 | Female | Site E | e8 | 30.5 | Female |
| Site B | b8 | 28 | Female | Site E | e9 | 30 | Female |
| Site B | b9 | 27.5 | Male | Site E | e10 | 29.5 | Female |
| Site B | b10 | 22.5 | Female | Site E | e11 | 28 | Male |
| Site C | c1 | 35.5 | Male | Site E | e12 | 27 | Female |
| Site C | c2 | 31 | Female | Site E | e13 | 25 | Male |
| Site C | c3 | 30 | Female | Site E | e14 | 25 | Female |
| Site C | c4 | 29 | Male | Site E | e15 | 24 | Female |
| Site C | c5 | 28.5 | Female |
Table 1: Total length (TL) and sex of observed individuals at the beginning of the observation.
Figure 3: Group distribution patterns in each site throughout the study period. Black lines indicate the home range of each individual. The habitat substrates are shown as gray lines. The open bar indicates 20 cm. In site E, e3 moved from group E2 to group E3 on 1 July 2015. As the hole of site E is quite deep, each group (E1, E2 and E3) is more than 80 cm distant from each other.
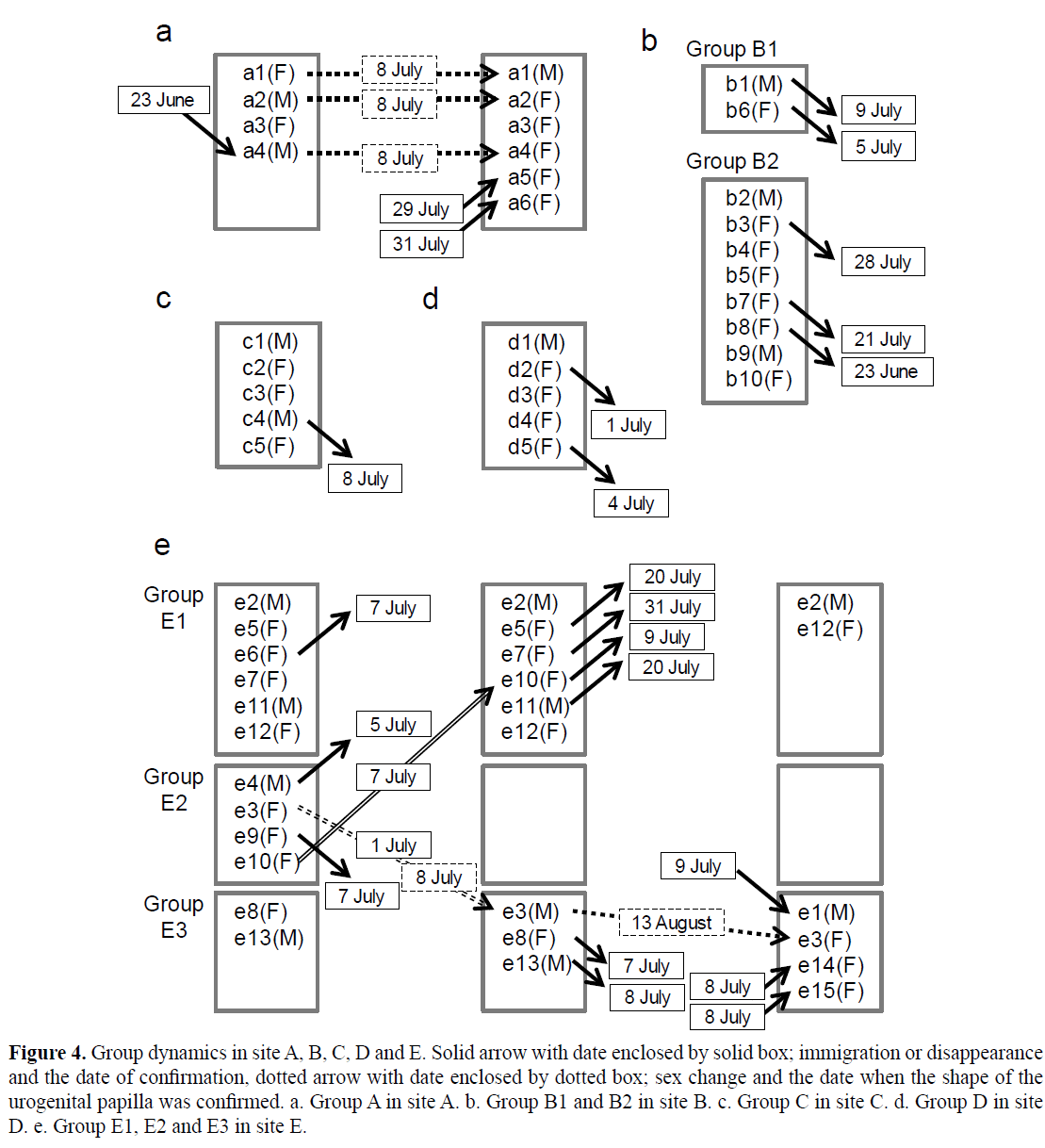
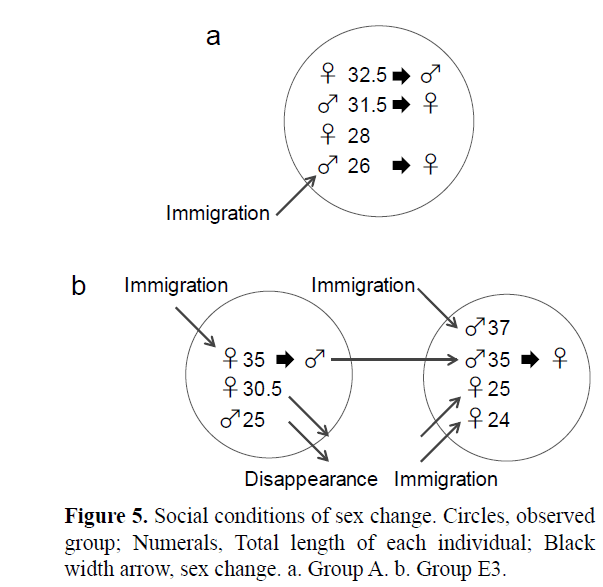
Figure 4: Group dynamics in site A, B, C, D and E. Solid arrow with date enclosed by solid box; immigration or disappearance and the date of confirmation, dotted arrow with date enclosed by dotted box; sex change and the date when the shape of the urogenital papilla was confirmed. a. Group A in site A. b. Group B1 and B2 in site B. c. Group C in site C. d. Group D in site D. e. Group E1, E2 and E3 in site E.
A total of 21 aggressive behaviors were observed among individuals in the same group except one instance: e2 of E1 attacked e13 of E3, which approached group E1. T. grammistes performed aggressive behavior by directed swimming toward stationary or moving conspecifics and attack by chasing, picking or biting. Although one aggressive behavior between same-sized individuals was recorded, all aggressive behaviors were performed by largest individuals in each group regardless of their sex: males and females performed aggressive behavior 15 and 6 times, respectively.
A total of 12 courtship behaviors were observed among same-group individuals. In T. grammistes, males performed courtship behaviors like T. okinawae (Sunobe and Nakazono, 1990): the male swims toward the female little by little and leads the female to the spawning nest. These observed courtship behaviors were performed 11 and one times by the largest male and the 5th largest male (e11) in group E1, respectively.
Spawning was observed 11 times. Spawning was taken place in small crevice and hole of the rock, and eggs were laid on their roof. In six cases, the spawning females were not identified, whereas five of the spawning females were identified as belonging to the same group as the spawning male: a1, e2, and b1 mated with a2, e12, and b6, respectively. d1 mated with d3 twice. All spawning males were identified as the largest male of their group. After spawning, the female left the nest and the male cared for the egg mass.
The largest males of each group performed courtship behavior toward, and spawning with 1-2 females (mean ± SD=1.5 ± 0.55) except males in which both courtship behavior and spawning were not observed, and cases in which we could not identify the spawning female.
Group dynamics and sex change
The social group dynamics are summarized in Figures 4 and 5. Of all observed individuals, 24 (7 males and 17 females) individuals moved their groups (including disappearance, immigration and movement between observed groups), whereas 17 (6 males and 11 females) individuals remained in their groups throughout study period. Although inter-group movement of individuals within the same site was observed, there was no case for the inter-site movement. We observed that individuals left the harem in 19 cases, and could and could not rediscovery in 2 and 17 cases, respectively. The GLM predicted no significant effect on movement of TL (coefficient=-0.0008, SE=0.122, Z=-0.007, P=0.995), sex (coefficient=-3.739, SE=5.948, Z=-0.629, P=0.53) and an interaction term between TL and sex (coefficient=0.127, SE=0.193, Z=0.658, P=0.511). There were no significant differences between the ratio of individuals that moved and those that remained in males (chi-square test, χ2=0.08, P>0.1, n=13) and females (chi-square test, χ2=1.29, P>0.1, n=28). At the end of the observation, group B1 and E2 had disappeared, and the group B2 contained two males and three females. The other groups comprised one male and one to five females (Figure 4).
Two and three instances of female-to-male and male-to-female sex change were confirmed during the study period, respectively, based on change in the shape of urogenital papilla. In group A, the largest female (a1) and the second largest male (a2) that had existed since the beginning of the observations changed sex to male and to female on 8 July, respectively (Figure 5a). In addition, the fourth largest male (a4), which immigrated to group A on 23 June, changed sex to female on 8 July (Figure 5a). After we confirmed sex change, a1 mated with a2 as male and as female respectively on 29 July.
In site E, e3 changed sex from female to male on 8 July after it became the largest individual in the social group by immigration from group E2 to group E3 on 1 July. Subsequently, a male (e1) that was larger than the resident male (e3) newly immigrated into group E3 on 9 July, and e3 changed its sex back to female on 13 August (Figure 5b).
In these cases, the female-to-male sex change was confirmed when the female remained in or immigrated to a social group consisting of smaller individuals than itself. The male-to-female sex change was confirmed when a male remained in or immigrated to the social group with larger individuals, or when the male’s size rank dropped as a result of immigration of a larger individual.
Discussion
Shiobara (2000) reported polygynous mating in T. grammistes from rearing experiments: the single largest male mated with some females. The present study showed that T. grammistes has harem polygyny under natural conditions based on following three observations. First, in most cases, T. grammistes lived in social groups with one large male and some females (Figure 4). Although the largest individuals in group A and E3 were female at the beginning of the observations, these groups also became group comprising one large male and some smaller females at the end of observation. Second, there was a social hierarchy based on body size in the social groups. Aggressive behavior was always observed from the largest individuals in each group toward smaller individuals. Third, although multiple males appeared in a group, the larger male monopolized mating opportunities.
The present study shows two and three instances of female-to-male and male-to-female sex change, respectively. Furthermore, one individual (e3) changed sex from female to male to female, sequentially (Figure 5). Although spawning was not observed before and after sex change by sex changers, we confirmed spawning by the male (a1) and female (a2) after they changed sex. It suggested that they changed sex functionally. As bidirectional sex change in T. grammistes has previously only been confirmed in aquarium (Shiobara, 2000), this is the 12th species report in which bidirectional sex change is confirmed in the field. The adaptive significance of bidirectional sex change in protogynous fish has been explained based on SA model (Nakashima et al., 1995; Munday et al., 2010). The SA model predicted that reversed sex change is favored when the sexspecific putative reproductive success depends on size (or age) reversal by fluctuation of social rank in harem polygynous species. The present results suggest that female to male and male to female sex change in T. grammistes usually takes place according to social rank as reported in T. okinawae (Sunobe and Nakazono, 1990; Manabe et al., 2007a). In this study, the largest female was likely to change sex to male to obtain more reproductive success than as a female by monopolizing mating opportunities with the females in same harem. In contrast, small males may change sex to female to prevent loss of reproductive success. These sex changes should contribute to maximizing the reproductive success of individuals in the harem polygyny. However, the cost of movement among spatially isolated habitat is also considered to be important to the evolution of bidirectional or reversed sex change in small goby, P. echinocephalus, G. histrio and T. okinawae (Nakashima et al., 1995; Munday, 2002; Manabe et al., 2007a). In addition, Sawada et al. (2017) predicted that that ecological condition (the high risk of movement and low population density) should favor reversed sex change over movement in protogynous and harem polygyny fish. Present study suggested that T. grammistes faces considerable risk of predation because although inter-group movement of individuals within the same hole (site E) was observed, there was no case for the inter-site movement. Their small body size (29.6 ± 3.2 mm TL in this study) may one of the reasons for those small moving range. Therefore, the risk of movement among spatially isolated harems seems to be an important factor in the evolution of reversed (male to female) sex change in T. grammistes.
Although it is considered that T. grammistes faces considerable risk of predation when they move among spatially isolated harems, individual movement (including disappearance, immigration and movement between harem) was observed frequently in this study. In T. grammistes, individuals moved their harem (20% of individuals moved their harem per month) more than twice as often as did T. okinawae (7.5 % per month) (Sunobe and Nakazono, 1990). This high frequency of movement in T. grammistes indicates that the structure and social rank in the harem may be unstable. Under these conditions, rapid sex change, which is a noted feature of the genus Trimma, should be adaptive to maximize reproductive success. It may also explain why a remarkably high frequency of reversed sex change was observed in this study compared with other polygynous species under natural conditions; in T. grammistes; 6.7% of males change sex to female per month, in T. okinawae; 0.4 % per month do so (Manabe et al., 2007a), and in Cirrhitichthys falco; 0.7% change sex to female per month (Kadota et al., 2012). Manabe et al. (2007b) suggested that females assess other harems and move to increase their probability of becoming a dominant male in T. okinawae. However, T. grammistes moves into/out of harems regardless of their sex and body size. In this study, although the instability of social structure was shown, we could not reveal the reason of it (e.g. the high frequency of movement among harem, high mortality rate, or both of them) because of the low rediscovery rate of individuals which left their harem (89% of individuals were not rediscovery after having left their harem). Further exhaustive investigation in locations where isolated harems are densely distributed is needed to confirm such a movement strategy.
The mating system and adaptive aspect of bidirectional sex change in T. grammistes were similar to those of T. okinawae (Sunobe and Nakazono, 1990; Sunobe and Nakazono, 1993; Manabe et al., 2007a). However, the sex–size distribution of T. grammistes was different from that of T. okinawae in that in the former, there was considerable overlap and small males were present (Figure 2). In fact, we observed that some small males remained in a harem without undergoing sex change. If a small male cannot obtain reproductive success because of interference or exclusion by large males, small males should change sex to female or emigrates to another harem. Do small males obtain any reproductive success? The appearance of small males has been confirmed in some polygynous and protogynous gobiid fish such as Lythrypnus dalli (St. Mary, 1993; Drilling and Grober, 2005), Fusigobius neophytus (Tsuboi and Sakai, 2016) and Coryphopterus nicholsi (Cole, 1983). Although there is little information about the reproductive behavior of such small male, Tsuboi and Sakai (2016) confirmed that, in F. neophytus, small males obtain some reproductive success using sneaking behavior. This indicates that there is a possibility that small males in protogynous species can obtain reproductive success. Although we have never observed spawning by small males, they are probably functional males because Shiobara (2000) confirmed the presence of functional testis in small males (14-32 mm SL), and we observed courtship behavior by a small male in this study. The cause for appearance of small males in this polygynous fish requires further investigation of reproductive status of small males, namely, whether or not small males have reproductive ability and opportunity.
Conclusion
Our underwater investigation revealed that T. grammistes lived in harems with one large male and some females. However, small males remained in some harems without sex change. Their reproductive status remains unknown. We also detected bidirectional sex changes in the field. As these sex changes took place according to fluctuation of social rank in the harem, the adaptive significance should be explained by the SA model. This is the first report of bidirectional sex change in T. grammistes under natural conditions.
Acknowledgements
The Ito diving service BOMMIE provided the facilities for the fieldwork. We are grateful to K. Shiota, N. Yamamoto, R. Tamaki, Y. Kazama and other staff of the above diving service for their encouragement during the study. Critical reading by two anonymous reviewers led the manuscript to significant improvement. This work was supported by JSPS KAKENHI Grants to T.S. (no. 19570016, 24370006 and 16K 07507) from the Japan Society for the Promotion of Science.
References
- Avise, J., 2011. Hermaphroditism: a primer on the biology, ecology, and evolution of dual sexuality. Columbia University Press, New York.
- Cole, KS., 1983. Protogynous hermaphroditism in a temperate zone territorial marine goby, Coryphopterus nicholsi. Copeia, 1983: 809-812.
- Cole, KS., 2010. Gonad morphology in hermaphroditic gobies. In: Reproduction and sexuality in marine fishes: patterns and processes. (Ed. Cole. KS). University of California Press, California, pp: 117-162.
- Drilling, CC., Grober, MS., 2005. An initial description of alternative male reproductive phenotypes in the bluebanded goby, Lythrypnus dalli (Teleostei, Gobiidae). Environ. Biol. Fish., 72: 361-372.
- Fukuda, K., Manabe, H., Sakurai, M., Dewa, S., Shinomiya, A., Sunobe, T., 2017. Monogamous mating system and sexuality in the gobiid fish, Trimma marinae (Actinopterygii: Gobiidae). J. Ethol., 35: 121-130.
- Ghiselin, MT., 1969. The evolution of hermaphroditism among animals. Q. Rev. Biol., 44: 189-208.
- Kadota, T., Osato, J., Nagata, K., Sakai, Y., 2012. Reversed sex change in the haremic protogynous hawkfish Cirrhitichthys falco in natural conditions. Ethology, 118: 226-234.
- Kuwamura, T., Nakashima, Y., 1998. New aspects of sex change among reef fishes: recent studies in Japan. Environ. Biol. Fish., 52: 125-135.
- Kuwamura, T., Kadota, T., Suzuki, S., 2014. Testing the low-density hypothesis for reversed sex change in polygynous fish: experiments in Labroides dimidiatus. Sci. Rep., 4: 4369.
- Kuwamura, T., Kadota, T., Suzuki, S., 2015. Bidirectional sex change in the magenta dottyback Pictichromis porphyrea: first evidence from the field in Pseudochromidae. Environ. Biol. Fish., 98: 201-207.
- Kuwamura, T., Nakashima, Y., Yogo, Y., 1994. Sex change in either direction by growth-rate advantage in the monogamous coral goby, Paragobiodon echinocephalus. Behav. Ecol., 5: 434-438.
- Kuwamura, T., Suzuki, S., Kadota, T., 2016. Male-to-female sex change in widowed males of the protogynous damselfish Dascyllus aruanus. J. Ethol., 34: 85-88.
- Manabe, H., Ishimura, M., Shinomiya, A., Sunobe, T., 2007a. Field evidence for bi-directional sex change in the polygynous gobiid fish Trimma okinawae. J. Fish. Biol., 70: 600-609.
- Manabe, H., Ishimura, M., Shinomiya, A., Sunobe, T., 2007b. Inter-group movement of females of the polygynous gobiid fish Trimma okinawae in relation to timing of protogynous sex change. J. Ethol., 25: 133-137.
- Manabe, H., Matsuoka, M., Goto, K., Dewa, S., Shinomiya, S., Sakurai, M., Sunobe, T., 2008. Bi-directional sex change in the gobiid fish Trimma sp.: does size-advantage exist? Behaviour, 145: 99-113.
- Manabe, H., Toyoda, K., Nagamoto, K., Dewa, S., Sakurai, M., Hagiwara, K., Shinomiya, A., Sunobe, T., 2013. Bidirectional sex change in seven species of Priolepis (Actinopterygii: Gobiidae). Bull. Mar. Sci., 89: 635-642.
- Munday, PL., 2002. Bi-directional sex change: testing the growth-rate advantage model. Behav. Ecol. Sociobiol., 52: 247-254.
- Munday, PL., Buston, PM., Warner, RR., 2006. Diversity and flexibility of sex-change strategies in animals. Trends. Ecol. Evol., 21: 89-95.
- Munday, PL., Kuwamura, T., Kroon, FJ., 2010. Bidirectional sex change in marine fishes. In: Reproduction and sexuality in marine fishes: patterns and processes. (Ed. Cole. KS). University of California Press, California, pp: 241-271.
- Nakashima, Y., Kuwamura, T., Yogo, Y., 1995. Why be a both-ways sex changer. Ethology, 101: 301-307.
- Sadovy de Mitcheson, Y., Liu, M., 2008. Functional hermaphroditism in teleosts. Fish. Fish., 9: 1-43.
- Sakurai, M., Nakakoji, S., Manabe, H., Dewa, S., Shinomiya, A., Sunobe, T., 2009. Bi-directional sex change and gonad structure in the gobiid fish Trimma yanagitai. Ichthyol. Res., 56: 82-86.
- Sawada, K., Yamaguchi, S., Iwasa, Y., 2017. Be a good loser: A theoretical model for subordinate decision-making on bi-directional sex change in haremic fishes. J. Theor. Biol., 421: 127-135.
- Shiobara, Y., 2000. Reproduction and hermaphroditism of the gobiid fish, Trimma grammistes, from Suruga Bay, central Japan. Sci. Rep. Mus. Tokai. Univ., 2:19-30
- Shitamitsu, T., Sunobe, T., 2016. Notes on protandry in the creediid fishes Limnichthys fasciatus and L. nitidus (Teleostei: Creediidae). Ichthyol. Res.
- St. Mary, CM., 1993. Novel sexual patterns in two simultaneously hermaphroditic gobies, Lythrypnus dalli and Lythrypnus zebra. Copeia, 1993: 1062-1072.
- Sunobe, T., Nakazono, A., 1990. Polygynous mating system of Trimma okinawae (Pisces: Gobiidae) at Kagoshima, Japan with a note on sex change. Ethology 84: 133-143.
- Sunobe, T., Nakazono, A., 1993. Sex change in both directions by alteration of social dominance in Trimma okinawae (Pisces: Gobiidae). Ethology 94: 339-345.
- Sunobe, T., Sado, T., Hagiwara, K., Manabe, H., Suzuki, T., Kobayashi, Y., Sakurai, M., Dewa, S., Matsuoka, M., Shinomiya, A., Fukuda, K., Miya, M., 2017. Evolution of bidirectional sex change and gonochorism in fishes of the gobiid genera Trimma, Priolepis, and Trimmatom. Sci. Nat., 104: 15.
- Suzuki, T., Yano, K., Senou, H., 2015 Trimma yoshinoi, a new gobiid fish from Japan (Perciformes: Gobiidae). J. Ocean. Sci. Found. 14: 66-73.
- Tsuboi, M., Sakai, Y., 2016. Polygamous mating system and protogynous sex change in the gobiid fish Fusigobius neophytus. J. Ethol. 34: 263-275.
- Warner, RR., 1975. The adaptive significance of sequential hermaphroditism in animals. Am. Nat. 109: 61-82.
- Warner, RR., 1984. Mating behavior and hermaphroditism in coral reef fishes. Am. Sci. 72: 128-136.
- Winterbottom, R., Hanner, RH., Burridge, M., Zur, M., 2014. A cornucopia of cryptic species- a DNA barcode analysis of the gobiid fish genus Trimma (Percomorpha, Gobiiformes). ZooKeys 381: 79-111.