Research Article - International Journal of Pure and Applied Zoology (2022) Volume 10, Issue 4
PARTICIPATION OF THE HYPOTHALAMIC-HYPOPHYSIAL NEUROSECRETORY SYSTEM IN THE OCCURRENCE OF A MIGRATION IMPULSE IN FISH
Garlov Pavel E*
Department of Neurology, Saint-Petersburg State Agrarian University, Saint-Petersburg, Russia
- *Corresponding Author:
- Garlov Pavel E
Department of Neurology
Saint-Petersburg State Agrarian University
Saint-Petersburg, Russia
E-mail: garlolv@mail.ru
Received: 27-Jan-2022, Manuscript No. IJPAZ-22-48579; Editor assigned: 31-Jan-2022, PreQC No. IJPAZ-22-48579(PQ); Reviewed: 15-Feb-2022, QC No. IJPAZ-22-48579; Revised: 18-Apr-2022, Manuscript No. IJPAZ-22-48579(R); Published: 25-Apr-2022, DOI:10.35841/2320-9585-10.4.116
Abstract
The participation of the Hypothalamic-Hypophysial Neurosecretory System (HHNS) in the initiation of anadromic fish migration was discovered. It is based on the results of ecological-histophysiological studies, using light microscopy, including immunohistochemistry, electron microscopy with quantitative morphometry. At the beginning of migration, an activation of neurosecretory products synthesis in pericarions of nonapeptidergic Neurosecretory Cells (NSC) and their transport to neurohypophysis where they accumulate occures. The excretion of neurosecretory products from pericarions of NSC into the liquor of the III brain ventricle is shown at the same time. We assume that HHNS causes a complex synchronous effect, which first consists of the active neurotropic action of Nonapeptide Neurohormones (NP-Nh) to the behavioral centers of the Central Nervous System (CNS), causing a dominant state of its excitation, designated as a "Migration Impulse". In contrast, the cessation of viscerotropic action of NP-Nh through the general blood flow in neurohypophysis causes both the violation of the longly adapted level of the marine "pastured" type of osmoregulation, and the interruption of the known anti-gonadotropic action of NP-Nh. And the latter contributes to the transition of the body to the energy-intensive energetical type of metabolism. In subsequent navigation mechanisms, widely covered in world literature, the leading role is played by the gonadoliberinergic forebrain centers.
Keywords
Hypothalamic-hypophysial neurosecretory system in fish, Neuroendocrine regulation mechanisms of sturgeon and salmon migrations, Migration impulse.
Introduction
Before our work it was known, that HHNS (or rather “preopticopost- neurohypophysial nonapeptidergic neurosecretory system”, most powerful in the neuroendocrine complex of the brain), which produces 2 nonapeptide neurohormones (NP-Nh: arginine-8-vasotocin, VT and isotocin, IT in bony fish), is responsible for regulation of the most important body functions: water-salt metabolism, tone of the gonads smooth muscles, spawning behavior, and that it is involved in stress reactions [1]. It was initially assumed that the leading role in determining migration behavior in fish is performed by HHNS. At the beginning of spawning migration in primary monocyclic forms anadromous lampreys and catadromous eels, as well as in anadromous sturgeons, salmons, down-stream migration of salmon smolts, clear changes in osmotic and ion regulation were established, but not in the functional state of HHNS.
However, during the period of spawning migrations, an inverse correlation was often noted in the content of Neurosecretory Material (NSM) in the central (Nucleus preopticus-NPo, synthetic center) and distal (neurohypophysis-NH, center of accumulation and extrusion of NSM) parts of HHNS, and also many large Neurosecretory Terminals (NT)-Herring's bodies (HB, up to 50 μm) appeared in NH [2]. Since it was not stated any patterns of HHNS functional changes during fish migrations, nature of the trigger mechanism of migrations, the so-colled "Migration Impulse", is not clear to date. Therefore, the only leading mechanism of fish migrations is considered to be navigational processes of the geomagnetic fields impact on the body receptor systems and olfactory imprinting and homing.
The purpose of the research is to find out the functional role of HHNS in the implementation of fish spawning migration [3-5]. The main objective is to determine the degree of HHNS participation in the process of anadromous fish spawning migration based on the ecological-histophysiological study.
Materials and Methods
Morpho-functional state of HHNS was studied in sexually matured producers of passing anadromous fish: springspawning Russian sturgeon Acipenser gueldenstaedtii, (stellate sturgeon) A. stellatus from the lower reaches of the Volga river and autumn-spawning monocyclic pink salmon Oncorhynchus gorbuscha from the lower reaches and spawning grounds of the Naiba and Umba rivers [6-8].
For light microscopy, brain tissue with hypophysis was fixed in Bouen fluid, histological sections (5-6 μm thick), were stained with Paraldehyde-Fuchsin (PAF) according to Gomori-Gabe’s method with Heidenhain’s azan staining. Functional activity of HHNS was determined by morphometric quantitative methods for assessing the degree of NSM content in NSC of Nucleus Preopticus (NPo) and in its accumulations in the proximal and distal parts of NH roots. Cytospetrophotometry of histological preparations was carried on the microanalyzers "Morpho- quant" and "Video-test", using the Programs "Ancell" and "Videotest".
For immunocytochemical research a peroxidase-antiperoxidase method for detecting unlabeled antibodies was carried out, and to detect Vasotocinergic (VT-) and Isotocinergic (IT-) structures were used antiserums to VT and mesotocin, which allows to identify VT- and IT-NSC [9]. Electron microscopical research was carried out on material fixed in glutaraldehyde, postfixed in tetroxide osmium (to Coulfield) and enclosed in araldite and epon. Ultrafine slices were contrasted (to Reynolds) in uranium acetate and lead citrate. The percentages ratios of NSC of dorsal part NPo (NPo magnocellularis) in different phases of their secretory and extrusion cycles (of neurosecretory terminals-NT of ?1, ?2, B types in NH) were analyzed. The results of quantitative morphometry of HHNS structures and ultrastructures are processed statistically using the Microsoft Excel program and presented in tables, histograms and diagrams [10-16].
The article applied an ecological-histophysiological approach, or method of scientific research in the form of the analysis adaptation mechanisms at various levels of organization, considered as the result of an experiment, set by nature itself, and its aim is to clarify the role of cellular and tissue structures in the implementation of the most important phylogenetic adaptations that ensure the biological progress of the specy [17-22]. So, for a constructive analysis the mechanism of HHNS participation in fish migrations at different stages of gonadal maturity (sgm: IV-at the lower river migration, before spawning; V sgm-at ovulation, spermiation) a formalized graphic method of comparative analysis (“crossanalysis” from the field of assessing the novelty of inventions, discoveries and literary sources) alternative functional states of HHNS was used.
Results
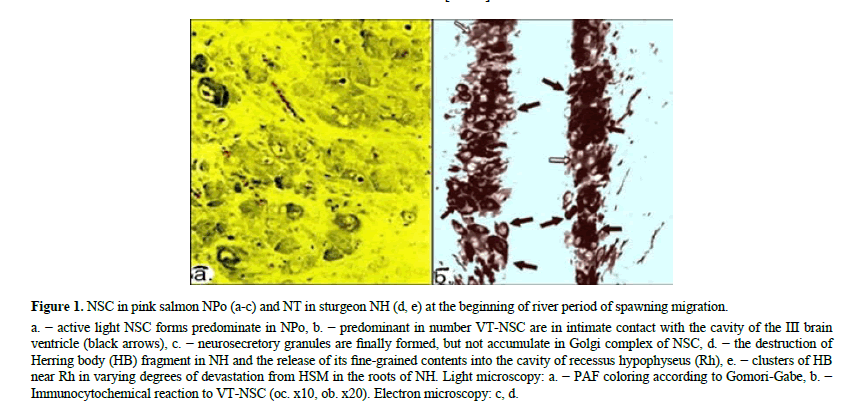
At the beginning of spawning migration, most NSC are represented by hypertrophied active "light" forms in secretory phases of "excretion" and "devastation from the NSM", while the vessels in the NPo region are also hypertrophied (Figure 1). By immunocytochemical study of NPo morpho-functional state it was revealed a significant predominance of VTimmunopositive NSC over IT-immunoreactive NSC (light arrows) [23-27]. In symmetrical parts of the NPo, many VTNSC apical surfaces are closely adjacent to cavity III of brain ventricle, forming a network of somato-ventricular (and dendroventricular) neurosecretory contacts in relation to IT- NSC 5:1. Electron microscopically, pictures of the mass formation of elementary neurosecretory granules (eng) and their direction to dendrites and axons are observed in Golgi complex [28-32]. In NPo prevail NSC in a state of high secretory activity, of 51%- 56% (Figure 2 and Table 1).
Figure 1: NSC in pink salmon NPo (a-c) and NT in sturgeon NH (d, e) at the beginning of river period of spawning migration.
a. − active light NSC forms predominate in NPo, b. − predominant in number VT-NSC are in intimate contact with the cavity of the III brain ventricle (black arrows), c. − neurosecretory granules are finally formed, but not accumulate in Golgi complex of NSC, d. − the destruction of Herring body (HB) fragment in NH and the release of its fine-grained contents into the cavity of recessus hypophyseus (Rh), e. − clusters of HB near Rh in varying degrees of devastation from HSM in the roots of NH. Light microscopy: a. − PAF coloring according to Gomori-Gabe, b. − Immunocytochemical reaction to VT-NSC (oc. x10, ob. x20). Electron microscopy: c, d.
| Quantitative relations of NSC in various functional states and phases of the secretory cycle in the dorsal part of NPo and the degree of its hyperemia* | |||||
|---|---|---|---|---|---|
| Stages of gonad maturity (IV, V sgm) | |||||
| Light microscopy | Electron microscopy | ||||
| Functional states of NSC | IV Spawning migration (in the lower reaches of the river) |
V At the beginning of spawning (on spawning graunds) |
Phases of NSC secretory cycle |
IV Spawning migration |
V At the beginning of spawning |
| 1. Rest | 8.2±1.74 | 5.8±1.77 | 1. Low or moderate activity | 8±0.81 | 3±0.24 |
| 2. Accumulation | 34.6±8.0 | 12.8±5.4 | 2. High activity | 42±5.43 | 59±8.53 |
| 3. Extrusion | 45.8±9.4 | 57.7±3.1 | 3. Deposit of NSM | 30±4.9 | 10±4.05 |
| 4. Exhaustion | 11.4±4.7 | 23.8±7.3 | 4. Hyperactivity | 9±1.92 | 21±3.47 |
| The width of the vessels lumen (degree of NPo hyperemia, μm) | 7.6±0.57 | 8.7±0.88 | 5. Reparations of cell organelles | 4±0.78 | 1±0.17 |
| 6. Mass organelles degradation | - | - | |||
| 7. Rest or deep braking functions | 7±1.22 | 6±1.34 | |||
Table 1. Morphometric characteristics of NSC in pink salmon NPo at the process of spawning migration and at the beginning of spawning.
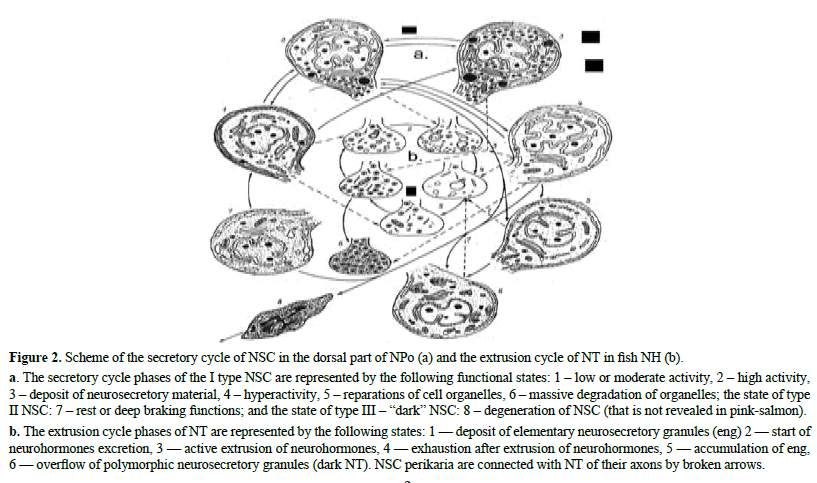
Figure 2: Scheme of the secretory cycle of NSC in the dorsal part of NPo (a) and the extrusion cycle of NT in fish NH (b).
a. The secretory cycle phases of the I type NSC are represented by the following functional states: 1 – low or moderate activity, 2 – high activity, 3 – deposit of neurosecretory material, 4 – hyperactivity, 5 – reparations of cell organelles, 6 – massive degradation of organelles; the state of type II NSC: 7 – rest or deep braking functions; and the state of type III – “dark” NSC: 8 – degeneration of NSC (that is not revealed in pink-salmon). b. The extrusion cycle phases of NT are represented by the following states: 1 — deposit of elementary neurosecretory granules (eng) 2 — start of neurohormones excretion, 3 — active extrusion of neurohormones, 4 — exhaustion after extrusion of neurohormones, 5 — accumulation of eng, 6 — overflow of polymorphic neurosecretory granules (dark NT). NSC perikaria are connected with NT of their axons by broken arrows.
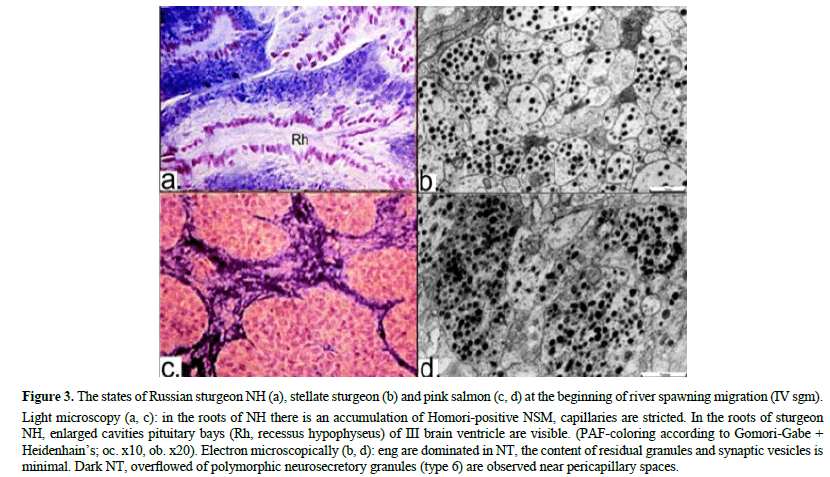
During period of active river spawning migration, mass accumulation of NSM occurs in NH (Figures 3 a,c), which is clearly consistent with the morpho-functional state of all its ultrastructures (Figures 3 b,d). NT, sinusoid capillaries and pituicytes in NH are in an inactive functional state, indicating unidirectionally to a low level (or absence) of NP-Nh excretion into the general bloodstream (Figure 3 and Table 2).
Figure 3: The states of Russian sturgeon NH (a), stellate sturgeon (b) and pink salmon (c, d) at the beginning of river spawning migration (IV sgm).
Light microscopy (a, c): in the roots of NH there is an accumulation of Homori-positive NSM, capillaries are stricted. In the roots of sturgeon NH, enlarged cavities pituitary bays (Rh, recessus hypophyseus) of III brain ventricle are visible. (PAF-coloring according to Gomori-Gabe + Heidenhain’s; oc. x10, ob. x20). Electron microscopically (b, d): eng are dominated in NT, the content of residual granules and synaptic vesicles is minimal. Dark NT, overflowed of polymorphic neurosecretory granules (type 6) are observed near pericapillary spaces.
| The content of NSM in NH, quantitative ratios of HT in different phases of the extrusion cycle (Figures 3 b), the degree of activity of pituitocytes and the degree of hyperemia of NG | |||||||
|---|---|---|---|---|---|---|---|
| Stages of gonad maturity (IV, V sgm) | |||||||
| Light Microscopy | Electron Microscopy*/ | ||||||
| Content of NSM in NH, state of NH structures (according to 5-point scale | IV Spawning migration (in the lower reaches of river) |
V At the beginning of spawning |
Phases of the extrusion cycle NT | IV Spawning migration |
V At the beginning of spawning |
||
| NSM concentration in the proximal NH divisions on the cytospectrophotometry results | |||||||
| NSM (in units of optical density) | 0.238±0.05 | 0.151±0.06 | |||||
| NSM concentration in the distal NH divisions on the visual results (according to 5-point scale) | ?1 | ?2 | ?1 | ?2 | |||
| NSM (points):? | 4.0±0.12 | 2.4±0.26 | 1. Deposit of eng | 17±2.35 | 7±0.53 | 7±2.86 | 2±1.05 |
| ? | 3.8±0.13 | 2.8±0.28 | 2. Start of NP-Nh excretion | 58±4.62 | 70±1.45 | 54±3.97 | 45±1.70 |
| ?+?(average) | 3.9±0.09 | 2.6±0.18 | 3. Active extrusion of NP-Nh | 20±3.58 | 20±1.94 | 33±8.44 | 47±2.87 |
| Diameter of pituicyte nuclei (μm) | 4.73±0.10 | 6.24±0.17 | 4. Exhaustion after extrusion of NP-Nh | 4±1.20 | 2±0.38 | 4±1.56 | 6±0.17 |
| Width of the capillaries lumen (μm) | 15.94±0,45 | 16.64±1.51 | 5. Accumulation of eng | 1±0.18 | 1±0.22 | 2±1.31 | - |
Table 2. Characteristics of structures and ultrastructures in distal parts of pink salmon NH at the process of spawning migration and at the beginning of spawning.
?lusters of HB in the roots of NH often occurs near the cavities of recessus hypophyseus (Rh), many of which are emptied from NSM and penetrate into it [33]. Pictures of destruction of the Herring body (HB) fragment by the type of macro-apocrine secretion, the disintegration of neurosecretory granules and the output of a fine-grained product into Rh (brain liquor) are revealed there. This indicates the active excretion of neurosecretory products from NSC into the liquor of the III ventricle not only in NPo region, but also in NH, in which, however, their excretion into the vessels of the general blood flow is absent [34].
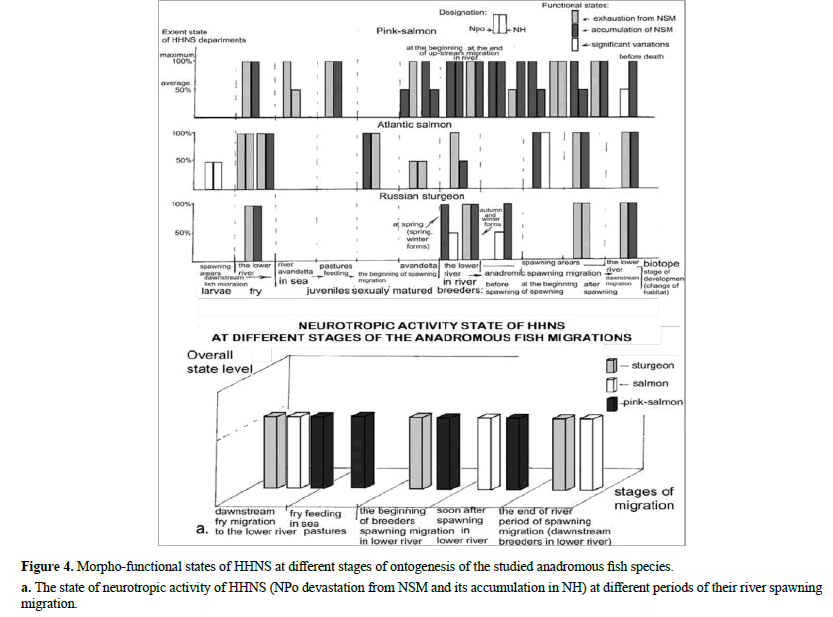
To verify our observations and in order to more objectively clarify the functional role of the HHNS in the implementation of migrations on more material, we used the method of comparative analysis of own and literary data. Specifically, in this work: 1) first of all, we formalized 2 alternative extreme states of the HHNS, according to the directions of NP-Nh excretion (and their unstable intermediate form), 2) then identified them in the literary sources, available to us (from primary: to the latest reviews: 3) analyzed in each work their sequence in ontogenesis, and 4) generalized all of them in a single histogram, based on their manifestation at the main stages of ontogenesis [35].
Accordingly, a comparative analysis of the HHNS function in alternative states (devastation, or accumulation of NSM in NPo and in NH, and significant variations in its content) at different stages of ontogenesis was produced, and the results are presented on histogram (Figure 4). This simplified, but clear dynamics of morphofunctional changes in the HHNS shows that the state of devastation of NPo from NSM, with its accumulation in NH, dominates during the process of spawning migrations of anadromous fish.
Discussion
The extreme morpho-functional states of HHNS in natural and experimental conditions, are mostly expressed either in the inverse correlation of the NSM content in its central (NPo) and distal (NH, neurohemal) parts or in the devastation of NH from the NSM. Accordingly, it is possible to distinguish 2 main states of HHNS functional activity, differing in the direction of the NP-Nh action and the achieved physiological effects:
The state of neurotropic activity of HHNS", characterized by NPo devastation from NSM and its accumulation in NH, which expresses the process of active synthesis of neurohormonal products in the NSC pericarions, their transport and excretion into the liquor of the III brain ventricle, where NP-Nh cause a neurotropic effect on the behavioral centers in the CNS [36]. State of viscerotropic activity of HHNS", in the form of NH devastation from NSM, which expresses the excretion of neurosecretory products from NT into the general blood circulation, where NP-Nh cause a viscerotropic effect on targetorgans. Such an activation of HHNS (at the organism level, in stress especially) has a generalized (multifunctional) and prolonged character.
Thus, it is shown that the "State of neurotropic activity of HHNS" is leading in the process of spawning migrations regardless of the season. NP-Nh transport to brain liquor is carried out from the apical part of NSC pericariorons in the regions of their somato- and dendro-ventricular neurosecretory contacts and from HB in the axo-ventricular contacts area in NH. Previously, an increase the synthetic activity of NP-NSC in the dorsal (magnocellular) part of NPo in females from the beginning of spawning migration and to spawning, especially VT-NSC at the pre-estuaries, and also of IT-NSC in the rostroventral (parvocellular) part of NPo in males before spawning was found in anadromic salmon species. However, the state of NH in the majority of these works was not considered. In Teleostei, NP-Nh affects centers of behavior regulation in the amygdal and hippocampal regions of the brain limbic system, causing "spawning reflex", where VT is more active (x10) than IT. VT receptors (V1) are found in the "Social Behavior Network" (or "Social Decision-Making Network"), regions responsible for social and sexual forms of behavior, where the effect of excitation these centers is the common. Thus, HHNS plays a key role in determining migration behavior, causing the dominant state of CNS excitation the form of "Migration Impulse", which we consider as a social form of behavior.
Another important part of the complex synchronous mechanism of HHNS participation in the anadromic migration is a significant decrease of its viscerotropic activity in the form of accumulation NSM in NH, above the "three-point" norm of moderate functioning in natural conditions [37]. This causes the violation of the long-term adapted “marine-pastured" hypotonic type of osmoregulation, due to the cessation of the active NP-Nh antidiuretic action, VT especially. The violation of the watersalt metabolism mechanisms in connection with the external medium salinity changes is evidenced by the progressive watering muscles in passage fish during migrations, especially in salmons. It is also assumed that VT and IT are involved in the body's ionic balance regulation through the gill blood-flow, since 2 different receptors (type V1) on the chloride-secreting cells of the gills respiratory epithelium in Teleostei were observed. Thus, the physiological stimulus of habitat change is a double effect caused by the HHNS, which consists in initiating persistent excitation of the "behavioral" centers of the CNS and in disrupting the long- adapted "pastured" hypotonic type osmoregulation and ionic balance of the body.
And a particularly important part of this starting neuroendocrine mechanism is the effect of violation anti- gonadotropic action of NP-Nh. It is carried out by direct inhibition of gonadoliberin (Gn-RH) secretion (synergy VT with dopamine), stimulation of adrenocorticotropin secretion in the adenohypophysis (synergy with corticoliberin), inhibition of endocrine and generative gonads functions, i.e. at all levels of the hypothalamichypophysial- gonadal axis. It is assumed, that the complex mechanisms of NP-Nh influence on reproductive processes are largely ensured by their interaction (VT, mainly) with adenohypophysiotropic hormones. The results of comparative tests of gonad-stimulating effects of the isolated anterior and posterior hypophysial lobes on sexual maturation and ovulation of sturgeon, sevruga and rotan (Perccottus glenii) showed, that NP-Nh extracts in physiological or slightly increased doses inhibit or disrupt ovulation process. The prolonged delay processes of ovulation and resorption sexual products of fishproducers in brackish water of "critical" salinity (4-8%) at spawning temperatures is also the effect of an increased content of NP-Nh in the blood [38]. Thus, HHNS, simultaneously with the starting neurotropic action, participates in triggering the activation functions all links of the hypothalamic-hypophysialgonadal axis. At the same time, the effects of viscerotropic NP-Nh action are generalized, since VT and IT receptors (type V1) are detected in many organs of Teleostei: pituitary, kidney, liver, ovary, gills, heart, muscles, spleen, lateral line, bladder, intestines. VT may be directly involved in the carbohydrate metabolism regulation, since its receptors (V1) are detected in fish hepatocytes and it was stated that NP-Nh stimulate glycogenolysis in trout and eel livers.
Therefore, we assume that this (the third, also synchronous) metabolic effect of NH-Nh, also generalized and prolonged, is the leading physiological mechanism for changing the plastic energy-saving metabolism to energy-intensive energetical [39].
Conclusion
One of the leading functions of HHNS is the generalized and prolonged effect of the synthesized NP-Nh on target organs, on which they have a stimulating action in small doses, and inhibitory in large doses. The physiologically dosed NP-Nh secretion and their functionally differentiated, specialized pathways excretion, determine their alternative effects- neurotropic and viscerotropic, especially metabolic, generalized and prolonged. Thus, the functional role of HHNS in fish migrations is to initiate migration processes in the form of "Migration Impulse" a complex synchronous effect of excitation brain behavioral centers, changing ion-osmotic body equilibrium and plastic metabolism to energical.
Further navigational mechanisms of fish migrations are provided by the close interaction of HHNS and diffuse accumulations of gonadoliberinergic (GnRH-) NSC, in which, for example, 2 forms of GnRH (salmon and chicken) in the ventral (smallcell) part of the NPo (Nucleus parvocellularis preopticus) modulate the electrical activity of VT-NSC in its large-cell part. The leading significance of each of them dynamically changes according to their functional role at different periods of reproduction. We suggest, that the dominant state of excitation ("Migration Impulse") is possibly a trigger involving primary phylogenetic navigational mechanisms of effecting geomagnetic fields on receptor systems and CNS, which are still not completely understood. In the further specialized navigation processes "olfactory" imprinting and homing, the main role is executed by GnRH- neurosecretory formations, localized near the "olfactory" and "visual" brain regions, where, GnRH, synthesized in olfactory nerve (in the organum vasculosum laminae terminalis), participates in the "navigational" processes of imprinting and homing, but which is synthesized in the preoptic region, provides the puberty and spawning processes in accordance with the biological significance of chemical and photoreception. The considered complex mechanism, determining migration behavior is the main reason for the progressive decrease in the eurybiontity degree of migratory fish in the process of spawning migrations and spawning.
Acknowledgement
I am grateful to my teachers, outstanding professors Nikolai Lvovich Gerbilsky and his follower Andrei Lvovich Polenov, founders of the national schools of ecological histophysiology and neuroendocrinology for friendly scientific education.
References
- Arvy, L., Fontaine, M., and Gabe, M., 1959. The hypothalamic-pituitary neurosecretory pathway of teleosts. Mason. J. Physiol., 51:1031-1085.
- Balment, R.J., Lu, W., Weybourne, E., and Warne, J.M. 2006. Arginine vasotocin a key hormone in fish physiology and behaviour: a review with insights from mammalian models. Gen. Comp. Endocrinol., 147: 9-16.
- Cooke, S.J., Hinch, S.G., Farrell, A.P., Patterson, D.A., Miller?Saunders, K., Welch, D.W., and Van der Kraak, G., 2008. Developing a mechanistic understanding of fish migrations by linking telemetry with physiology, behavior, genomics and experimental biology: an interdisciplinary case study on adult Fraser River sockeye salmon. Fisheries., 33: 321-339.
- Foran, C.M., and Bass, A.H., 1999. Preoptic GnRH and AVT: axes for sexual plasticity in teleost fish. Gen. Comp. Endocrinol., 116: 141-152.
- Garlov, P.E., 2005. Plasticity of nonapeptidergic neurosecretory cells in fish hypothalamus and neurohypophysis. Int. Rev. Cytol., 245: 123-170.
- Subasinghe, R., Soto, D., and Jia, J., 2009. Global aquaculture and its role in sustainable development. Rev. Aquac., 1: 2-9.
- Dolomatov, S.I., Zukow, W., Novikov, N.Y., Muszkieta, R., Bulatowicz, I., Dzierzanowski, M., and Strojek, K., 2012. The regulation of osmotic and ionic balance in fish reproduction and in the early stages of ontogeny. Russ. J. Mar. Biol., 38: 365-374.
- Sorensen, P.W., Fine, J.M., Dvornikovs, V., Jeffrey, C.S., Shao, F., Wang, J., and Hoye, T.R., 2005. Mixture of new sulfated steroids functions as a migratory pheromone in the sea lamprey. Nat. Chem. Biol., 1: 324-328.
- Godwin, J., and Thompson, R., 2012. Nonapeptides and social behavior in fishes. Horm. Behav., 61: 230-238.
- Goodson, J.L., and Bass, A.H., 2001. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res. Rev., 35: 246-265.
- Gozdowska, M., Kleszczy?ska, A., Soko?owska, E., and Kulczykowska, E., 2006. Arginine vasotocin (AVT) and isotocin (IT) in fish brain: diurnal and seasonal variations. Comp. Biochem. Physiol. B., 143: 330-334.
- Acher, R., and Chauvet, J., 1988. Structure, processing and evolution of the neurohypophysial hormone-neurophysin precursors. Biochimie., 70: 1197-1207.
- Forsling, M.L., Stoughton, R.P., Zhou, V., Kelestimur, H., and Demaine, C., 1993. The role of the pineal in the control of the daily patterns of neurohypophysial hormone secretion. J. Pineal Res., 14: 45-51.
- Hasunuma, I., Toyoda, F., Okada, R., Yamamoto, K., Kadono, Y., and Kikuyama, S., 2013. Roles of arginine vasotocin receptors in the brain and pituitary of submammalian vertebrates. Int. Rev. Cell Mol. Biol., 304: 191-225.
- Hiraoka, S., Ando, H., Ban, M., Ueda, H., and Urano, A., 1997. Changes in expression of neurohypophysial hormone genes during spawning migration in chum salmon, Oncorhynchus keta. J. Mol. Endocrinol., 18: 49-55.
- Kudo, H., Hyodo, S., Ueda, H., Hiroi, O., Aida, K., Urano, A., and Yamauchi, K., 1996. Cytophysiology of gonadotropin-releasing-hormone neurons in chum salmon (Oncorhynchus keta) forebrain before and after upstream migration. Cell Tissue Res., 284: 261-267.
- Kulczykowska, E., 2019. Arginine vasotocin and isotocin: Towards their role in fish osmoregulation. Fish Osmoreg., 151-176.
- Lema, S.C., 2010. Identification of multiple vasotocin receptor cDNAs in teleost fish: sequences, phylogenetic analysis, sites of expression, and regulation in the hypothalamus and gill in response to hyperosmotic challenge. Mol. Cell Endocrinol., 321: 215-230.
- Lohmann, K.J., Putman, N.F., and Lohmann, C.M., 2008. Geomagnetic imprinting: A unifying hypothesis of long-distance natal homing in salmon and sea turtles. Proc. Natl. Acad. Sci., 105: 19096-19101.
- Makino, K., Onuma, T.A., Kitahashi, T., Ando, H., Ban, M., and Urano, A., 2007. Expression of hormone genes and osmoregulation in homing chum salmon: a minireview. Gen. Comp. Endocrinol., 152: 304-309.
- Marshall, W.S., 2003. Rapid regulation of NaCl secretion by estuarine teleost fish: coping strategies for short-duration freshwater exposures. Biochim. Biophys. Acta. Biomembr., 1618: 95-105.
- Maximino, C., Lima, M.G., Oliveira, K.R.M., Batista, E.D.J.O., and Herculano, A.M., 2013. “Limbic associative” and “autonomic” amygdala in teleosts: a review of the evidence. J. Chem. Neuroanat., 48, 1-13.
- Kachynski, A.V., Kuzmin, A.N., Nyk, M., Roy, I., and Prasad, P.N., 2008. Zinc oxide nanocrystals for nonresonant nonlinear optical microscopy in biology and medicine. J. Phys. Chem. C., 112: 10721-10724.
- O’Connell, L.A., and Hofmann, H.A., 2012. Evolution of a vertebrate social decision-making network. Sci., 336: 1154-1157.
- Olson, K.R., 2002. Gill circulation: regulation of perfusion distribution and metabolism of regulatory molecules. J. Exp. Zool., 293: 320-335.
- Pierantoni, R., Cobellis, G., Meccariello, R., and Fasano, S., 2002. Evolutionary aspects of cellular communication in the vertebrate hypothalamo–hypophysio–gonadal axis. Int. Rev. Cytol., 218: 69-143.
- Polenov, A.L., and Garlov, P.E., 1972. The hypothalamo-hypophysial system in Acipenseridae. J. Cell Sci., 116: 349-374.
- Polenov, A.L., and Garlov, P.E., 1974. The hypothalamo-hypophysial system in acipenseridae-IV. The functional morphology of the neurohypophysis of Acipenser guldenstadti Brandt and Acipenser stellatus Pallas after exposure to different salinities. Cell Tissue Res., 148: 259-275.
- Polenov, A.L., Garlov, P.E., Koryakina, E.D., and Faleeva, T.I., 1976. The hypothalamo-hypophysial system in Acipenseridae. V. Ecological-histophysiological analysis of the neurohypophysis of the female sturgeon Acipenser guldenstadti Brandt during up-stream migration and after spawning. Cell Tissue Res., 170: 113-128.
- Polenov, A.L., Pavlovic, M., and Garlov, P.E., 1972. Preoptic nucleus and neurohypophysis in sturgeons (Acipenser guldenstadti Brandt) at different stages of their life cycle and in experiments. Gen. Comp. Endocrinol., 18: 617-617.
- Putman, N.F., Jenkins, E.S., Michielsens, C.G., and Noakes, D.L., 2014. Geomagnetic imprinting predicts spatio-temporal variation in homing migration of pink and sockeye salmon. J. R. Soc. Interface., 11: 20140542.
- Rose, J.D., and Moore, F.L., 2002. Behavioral neuroendocrinology of vasotocin and vasopressin and the sensorimotor processing hypothesis. Front. Neuroendocrinol., 23: 317-341.
- Saito, D., Hasegawa, Y., and Urano, A., 2003. Gonadotropin-releasing hormones modulate electrical activity of vasotocin and isotocin neurons in the brain of rainbow trout. Neurosci. Lett., 351: 107-110.
- Sternberger, L.A., and Joseph, S.A., 1979. The unlabeled antibody method. Contrasting color staining of paired pituitary hormones without antibody removal. J. Histochem. Cytochem., 27: 1424-1429.
- Ueda, H., 2012. Physiological mechanisms of imprinting and homing migration in Pacific salmon Oncorhynchus spp. J. Fish Biol., 81: 543-558.
- Ueda, H., 2019. Sensory mechanisms of natal stream imprinting and homing in Oncorhynchus spp. J. Fish Biol., 95: 293-303.
- Urano, A., and Ando, H., 2003. Quantitative analyses of the levels of hormonal mRNAs in the salmon neuroendocrine system. Aqua. Gen. Spr., 225-235.
- Warne, J.M., Harding, K.E., and Balment, R.J., 2002. Neurohypophysial hormones and renal function in fish and mammals. Comp. Biochem. Physiol. B., 132: 231-237.
- Zohar, Y., Munoz-Cueto, J.A., Elizur, A., and Kah, O., 2010. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol., 165: 438-455.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref