- International Journal of Pure and Applied Zoology (2016) Volume 4, Issue 2
Long-Term Sedentary Behavior Of Coconut Crabs In Their Northernmost Range
| S. Oka*, K. Miyamoto, and S. Matsuzaki Okinawa Churashima Foundation, 888 Ishikawa, Motobu, Okinawa 905-0206, Japan |
| Corresponding Author: S. Oka Okinawa Churashima Foundation 888 Ishikawa, Motobu Okinawa 905-0206, Japan E-mail: sh-oka@okichura.jp |
| Received 01st April 2016; Accepted 19th April 2016; Published 29th April 2016 |
Abstract
Movement of the coconut crab Birgus latro was investigated at the Ocean Expo Park in Okinawa Island, Japan, the northernmost range of this large, terrestrial crustacean. Recapture data were obtained using photographic matching of carapace groove patterns to identify individuals. Of the 485 crabs captured and photographed, 122 were recaptured and the distances they moved were recorded during an eight-year study period. Monthly changes in the sex ratios of crab populations at shore and inland areas indicated that females and a few males moved to the shore area during the reproductive season. On average, 69% of males and females did not move more than 200 m from their release points regardless of the duration of the liberty period. Long distance movements were not common. Differences in size and sex did not correlate with the distance moved. These observations indicate that coconut crabs have a highly sedentary lifestyle and that they remain in their home ranges for many years while repeating their homing behavior. The data suggest that coastal forests, which provide a corridor for migration, are important for crab conservation.
Keywords |
||||||||||||||
| Robber Crab, Migration, Capture-Recapture, Natural Marks, Individual Identification. | ||||||||||||||
INTRODUCTION |
||||||||||||||
| The coconut crab, Birgus latro (Linnaeus, 1767), is widely distributed in the tropical Indo-Pacific region (Drew et al., 2010). Populations of this species are declining in many of its island habitats, probably because of habitat loss and harvesting for human consumption (Drew et al., 2010). This species was listed globally as vulnerable in 1981 under the IUCN Red List. In 1996, the listing was downgraded to the data deficient category, not because the species had recovered but simply due to the lack of available data (Eldredge, 1996). The need for appropriate management strategies for the coconut crab has led to several studies on the ecology of this species (Drew et al., 2010). | ||||||||||||||
| Knowledge about migration patterns and the home range of animals is essential for resource management. The movement of coconut crabs has been investigated using the mark-recapture method (Fletcher et al., 1990), radio tagging (Fletcher et al., 1990; Schiller et al., 1991), GPS tagging (large males only, Krieger et al., 2012), and analysis of sex ratios (Sato and Yoseda, 2013). During the breeding season, female coconut crabs migrate from inland areas to the coast to lay fertilized eggs on the shoreline (Schiller et al., 1991). Males also migrate temporarily to the coast for mating, to drink saltwater, and to forage (Krieger et al., 2012; Sato and Yoseda, 2013). They maintain a sedentary existence in burrows during the day, and exhibit excellent homing instincts (Fletcher et al., 1990; Krieger et al., 2012). Although useful data were collected in the aforementioned studies, individual tracking was conducted for a relatively short period (a maximum of one year). Because coconut crabs can be remarkably long lived (more than 50 years) (Drew et al., 2013; Sato et al., 2013; Oka et al., 2015), it is important to examine their habits over a period of several years to understand the migratory and sedentary aspects of their natural history. | ||||||||||||||
| In our previous studies (Oka et al., 2014, 2015), we identified the following aspects of the coconut crab lifestyle in the northernmost population: crabs were observed frequently around coastal forests, which contained potential bait plants; crabs were not found when air temperature and humidity were less than 19°C and 58%, respectively; the reproductive season occurred between July and August; crabs survive and molt during winter in limestone caves in the coastal forests; maximum sizes of males and females are 68.8 mm and 47.5 mm thoracic length, respectively; and longevity is approximately 50 years for both sexes. Our previous study (Oka et al., 2013) also suggested that the pattern of grooves on the coconut crab carapace remain unchanged over several molting cycles, indicating that photographic matching of groove patterns can serve as a useful and simple technique for long-term individual identification. Here, we report the movement of coconut crabs based on recapture data obtained using photographic matching during an eight-year field survey on Okinawa Island in southern Japan. | ||||||||||||||
MATERIALS AND METHODS |
||||||||||||||
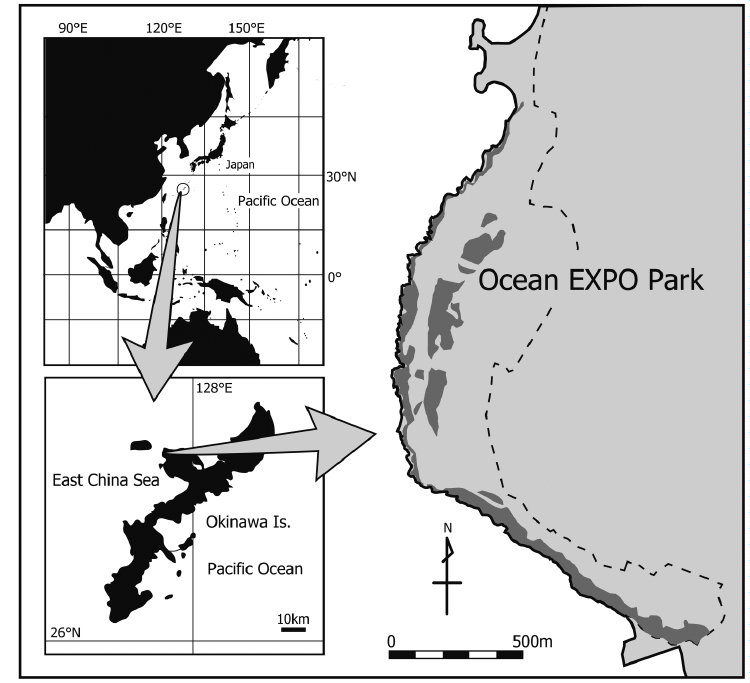
| A field investigation was conducted at the Ocean Expo Park (26°41′N, 127°52′E) on Okinawa Island, Japan, which has been managed by the Japanese government since 1976 (Figure 1). The park faces the East China Sea and has an area of 0.77 km2, with a coastline of 2.4 km. The park has some attractions (the aquarium, the botanical gardens, and the cultural museum etc.) on the inland side. Natural coastal forests are present continuously along the coast, with indigenous and introduced flora scattered throughout the inland region. The ground surface is primarily composed of coral limestone, and several erosional caves are present, especially in the coastal forest. The inland border of the park is divided by a fence that impedes the migration of coconut crabs. Coconut crabs are mainly observed close to the coastal forests on which they depend for prey and nest sites and few crabs have been found outside the park (Oka et al., 2014). This population may thus be regarded as semi-enclosed. Killing of animals, including coconut crabs, is prohibited by the official rules of the park. | ||||||||||||||
| Crabs were captured manually at night by searching on foot in the park from July 2006 to October 2014. Each night search was performed between one hour after sunset and midnight. The thoracic length (ThL) (see Fletcher et al., 1990) of the captured crabs was measured to the nearest 0.1 mm using vernier calipers, and the sex of each individual was determined. A dorsal view of the front cephalothorax of each crab was photographed using a digital camera to enable subsequent identification. After being measured and photographed, crabs were released immediately at the capture points. The capture points were recorded on the detailed blank map (scale: 1/5000), and longitude and latitude were determined using a portable GPS device. The capture points were classified as “shore area,” which faced the sea, and “inland area,” which was on the inland side of the coastal forest. | ||||||||||||||
| Identification of recaptured individuals was carried out by visual matching of photographs following the procedure in our previous report (Oka et al., 2013). The distance moved during the liberty period (the number of days between release and recapture) was defined as the linear distance between the release and recapture points. The distance is not the total distance to migrate during the liberty period. The distances were measured using the distance measurement function of Google Earth for Windows (Google) based on the data of the capture and recapture points. | ||||||||||||||
| Distance moved per day was calculated by dividing the linear distance between the release and recapture points by the liberty period in days. Only recapture data collected before wintering were used to eliminate the influence of negative activity during the winter season when the temperature was below 19°C (Oka et al., 2014). | ||||||||||||||
RESULTS |
||||||||||||||
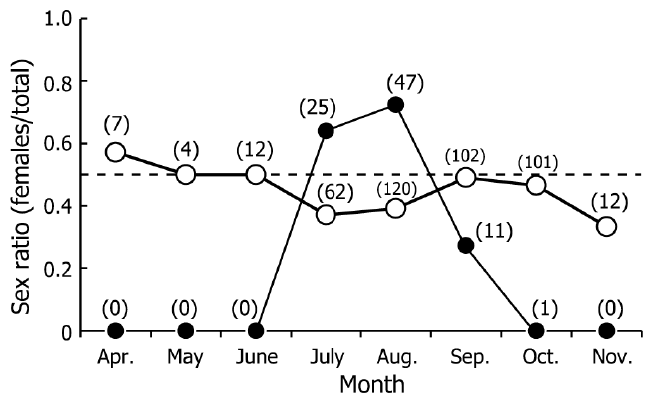
| During eight field seasons (160 surveys), a total of 264 males (16.4–68.8 mm ThL) and 221 females (14.3–47.5 mm ThL) were found. Figure 2 shows monthly changes in sex ratios in the shore area and inland area. Crabs were only found in the shore area between July and September. The sex ratio in the shore area between July and August was biased toward females (0.64–0.72). Sex ratios at the inland area were constant through most of the year, with the exception of July, August, and November, when capture rates of females were lower than those of males. | ||||||||||||||
| In total, 122 (62 males and 60 females) of the 485 crabs photographed were recaptured and the distances moved were calculated. Half of the crabs were recaptured once (n = 61, 30 males and 31 females), 22 (11 males and 11 females) were recaptured twice, 3 (all females) were recaptured three times, and 3 (all males) were recaptured four times. The liberty period ranged from 2 to 2384 days in males and from 2 to 2175 days in females. No significant difference in the liberty period was observed between the sexes (Mann–Whitney U–test, p >0.05). | ||||||||||||||
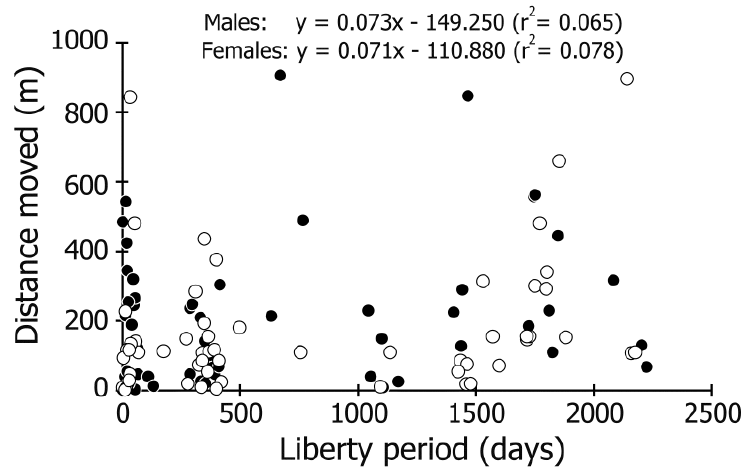
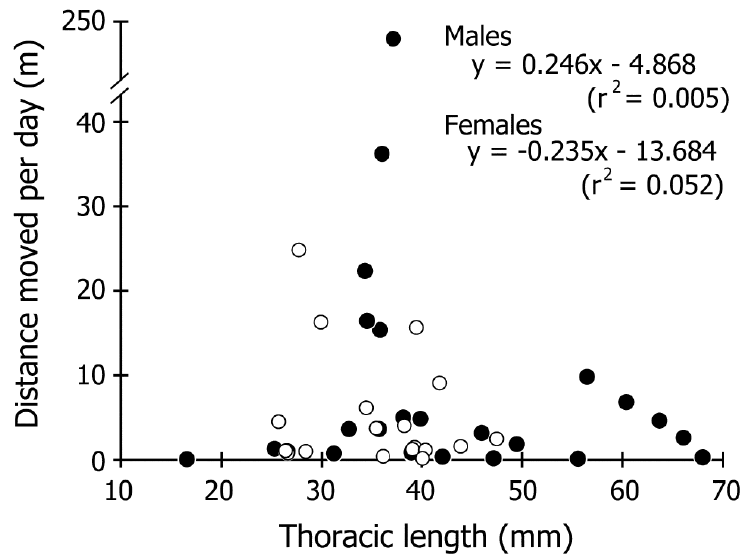
| The distance moved ranged from 2 to 906 m, and 52%– 85% (69% on average) of males and females in each liberty period group (defined by the number captured prior to winter, or wintering counts, which indicate the number in the active period except for wintering season) were recaptured within 200 m of their release point (Figure 3). No correlation was detected between the liberty period and distance moved in either sex (r2<0.08; Figure 4). The most rapid movement was 242.5 m/day (Figure 5). No correlation was found between size and distance moved per day of crabs that were recaptured before wintering (r2<0.06; Figure 5). | ||||||||||||||
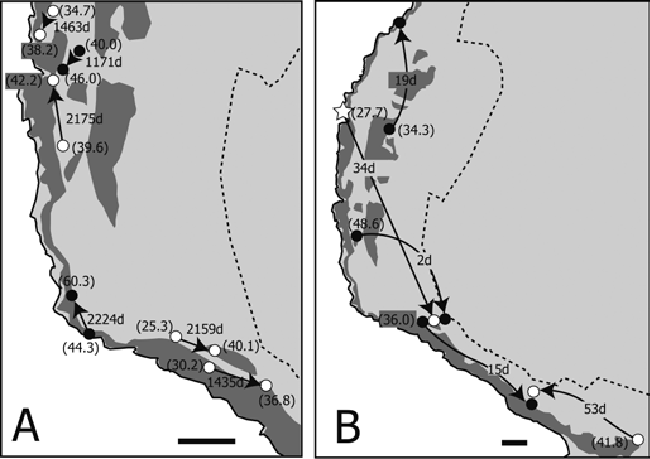
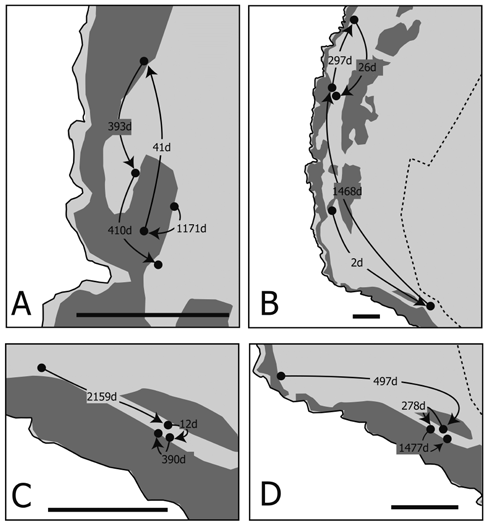
| Individual movement patterns were loosely classified into short-migration (less than 100 m of movement during a long period; Figure 6A) and rapid-long migration (more than 400 m of movement in a short period; Figure 6B). Both movement patterns were found in both sexes (Figure 6). A portion of the tracking record of crabs recaptured more than three times is shown in Figure 7. Most of the crabs were found within a 100 m radius regardless of their liberty periods (Figures 7A,7C and 7D). Although one crab migrated long distances (first recapture: 485 m in 2 days; second recapture: 847 m in 1,468 days; third recapture: 248 m in 297 days; and final recapture: 225 m in 26 days), the locations of the second and final recapture were close (Figure 7B). All crabs were found in and around the natural forest area (Figures 6 and 7). | ||||||||||||||
DISCUSSION |
||||||||||||||
| The monthly changes in the sex ratio indicate female migration to the shore area between July and September (Figure 2). The dominant female period (July to August) for the shore area coincided with their reproductive season, as reported previously (Oka et al., 2014). Females extrude fertilized eggs into limestone crevices in the shore area (Sato and Yoseda, 2009). The mean egg incubation period is estimated to be approximately 30 days (Schiller et al., 1991). Thus, female migration during the reproductive season in the northernmost area would is related to mating, incubation of eggs, and production of larvae, as shown in previous studies (Schiller et al., 1991; Sato and Yoseda, 2013). Temporary migration to the coastal area was also found among males. Males were found in the shore area only between July and September, and the total number was less than the total number of females (Figure 2). Male movement during the reproductive season is related to mating, drinking saltwater, and foraging, and has been reported previously using GPS telemetry (Krieger et al., 2012) and spatial changes in sex ratios (Sato and Yoseda, 2013). In the present study, the occurrence of males in the shore area during July and August is likely related to reproduction (Figure 2). | ||||||||||||||
| The resolution of our capture-recapture datasets was too low to estimate the detail of daily movements because it could only connect capture and recapture points after an arbitrary liberty period. However, we suggest that the data on recapture points might indicate the changing home range, considering that the crabs have a strong homing capability (Fletcher et al., 1990; Krieger et al., 2012). Our recapture data showed that, on average, 69% of the crabs did not move more than 200 m, and movements over 300 m were very infrequent, regardless of the liberty period (Figures 3, 4). Furthermore, differences in size and sex were not related to the distances moved (Figure 5). These findings indicate that coconut crabs have a sedentary lifestyle in which they stay in their home ranges for years while repeating their homing behavior. Previous studies in Vanuatu (Fletcher et al., 1990) and Christmas Island (Krieger et al., 2012) showed similar results, although the crabs were tracked for only short periods (less than one year) in these studies. Krieger et al. (2012) tracked large male crabs, while Fletcher et al. (1990) did not mention the differences between sexes. To our knowledge, this is the first study to clarify the long sedentary period and migration within each sex and among different sizes of crab over a period of several years despite intermittent data. | ||||||||||||||
| Although coconut crabs are usually highly sedentary, a number of individuals moved long distances (Figures 3, 6B, 7B). Long-distance movement was not related to liberty period, size, or sex (Figures 3-5). One male crab recaptured four times moved long distances between every recapture (Figure 7B). Although this is an extraordinary case, and contrary to the sedentary disposition of this species, these long-distance movements indicate that this crab changed his home range during the liberty period. Krieger et al. (2012) reported long-distance movement in males of a Christmas Island population; however, the reason for their long-distance movement was not determined. They suggested that longdistance movement was likely related to mating, drinking saltwater, and foraging. Our results show that some crabs did not protect their home range, and sometimes abandoned their sedentary lifestyle for unknown reasons. | ||||||||||||||
| Coconut crabs were found at the highest area of Christmas Island where there is rainforest cover from the coast to the inland area (Drew et al., 2010). In the Ocean Expo Park, most of the crabs were found around coastal forests, which are continuous along the coastline (Oka et al., 2013). This geographical situation might cause restriction of movement from the coastal forest to the inland area and/or outside of the park. However, the movement along the coastal forest was not restricted, because crabs could move at least 2.5 km through the continuous coastal forest (Figure 1). Thus, their sedentary lifestyle in the park was related not only the geographical situation but also to other resources (e.g. food, caves, encountering other crabs). Further study is necessary to clarify the important factors that affect their movement behavior. Their highly sedentary lifestyle indicated that the forests provide a corridor for crab migration, indicating the importance of forests for crab conservation. Taking into consideration the highly sedentary lifestyle of coconut crabs, then establishing sanctuaries will make conservation efforts more effective. | ||||||||||||||
CONCLUSION |
||||||||||||||
| This study determined the long-term movement of coconut crabs using recapture data on 122 individuals collected over an eight-year-study period. Identities of recaptured crabs were confirmed using photographic matching of carapace groove patterns. Although the crabs moved to the shore area during the reproductive season, the majority of the crabs did not move more than 200 m regardless of the liberty period. These results indicate the highly sedentary lifestyle of coconut crabs. | ||||||||||||||
ACKNOWLEDGMENTS |
||||||||||||||
| We thank the staff of the Okinawa Churaumi Aquarium for assisting us with the night searches; special thanks to Koji Tokutake, who made a substantial contribution. We thank Maya Iriondo for the language support in this article. The field research was conducted with the permission of the Okinawa Commemorative National Government Park Office. | ||||||||||||||
Figures at a glance |
||||||||||||||
|
||||||||||||||
References
|