Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2023) Volume 13, Issue 17
In-vitro evaluation of anticancer potential of vanillin-zinc herbomineral complex.
Heena Maithania*, S Patil Swati
Department of Pharmacognosy and Phytochemistry, Principal KM Kundnani College of Pharmacy, Mumbai, India
- Corresponding Author:
- Heena Maithania
Department of Pharmacognosy and Phytochemistry,
Principal KM Kundnani College of Pharmacy,
Mumbai,
India,
E-mail: heena.maithaniaict@gmail.com
Received: 18-Oct-2022, Manuscript No. AABPS-21-44831; Editor assigned: 21-Oct-2022, PreQC No. AABPS-21-44831 (PQ); Reviewed: 04-Nov-2022, QC No. AABPS-21-44831; Revised: 15-Feb-2023, Manuscript No. AABPS-21-44831 (R); Published: 15-Mar-2023, DOI: 10.35841/2249-622X.99.171
Citation:Maithania H, Swati SP. In-vitro evaluation of anticancer potential of vanillin-zinc herbomineral complex. Asian J Biomed Pharmaceut Sci. 2023;13(99):171
Abstract
The anticancer potential of phenolic constituents is mainly attributed to its free radical scavenging activity. However, the poor bioavailability exhibited my most phytoconstituents poses significant constraints for its in-vivo absorption, thereby limiting its anticancer activity. The herbomineral complex of vanillin with zinc was formed using the process of chelation. Zn (II) readily chelates with vanillin to form a stable water soluble vanillin-zinc complex with improved bioavailability. The complex formation was evaluated using UV-Vis spectroscopy, IR, Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES), NMR and mass spectroscopy. Vanillin-zinc complex revealed significant improvement in anticancer potential compared with vanillin.
Keywords
Vanillin, Herbomineral complex, Bioavailability, Phytoconstituent, Zinc, Anti cancer, Chelation.
Introduction
Plant derived agents have played a vital role in the treatment of cancer. Hemidesmus indicus (Apocynaceae), commonly known as Indian sarsaparilla is used in poly herbal preparations aimed against cancer, the root is mostly used in traditional medicine preparations. Vanillin, the major phytoconstituent of Hemidesmus indicus is known to posses good antioxidant potential. Antioxidant properties can result from their free radical scavenging activity but major drawback of phenolic compound is their poor bioavailability. In order to improve the bioactivity of the phytoconstituents, metal complexes are formed using chelation. Chelation involves the formation of two or more separate coordinate bonds between a polydentate ligand and a single central atom. The essential and characteristic feature found in all chelates is formation of a ring between the ligand and the metal atom. For ring formation to occur, the electron donor molecule containing two or more groups combines with the metal atom resulting in formation of at least one coordinate covalent bond [1].
Herbomineral complex formed by chelation involves the ligands as the active phytomolecule, forming the complex with the central metal ion. A biologically active metal complex with high thermodynamic stability thus releases the metal to the active site. The kinetics with which the metal ion undergoes ligation or de ligation is of great importance. The molecular weight of the metal complex is also critical. The compounds of low molecular weight with neutral charge and some water solubility are soluble in almost any medium and may be absorbed through membranes through passive diffusion. Herbomineral complex plays an important role in bioenhancement due to its manifold advantages like improved cell function, reduced free radical levels, improved blood flow, protection of nutritional value and increased antioxidant activity [2].
Vanillin has necessary atoms and groups to function as electron donors for formation of covalent bonds with Zn which is needed to form stable chelate. The hydroxyl oxygen of vanillin donates two electrons to form a covalent bond between this oxygen and metal. Further, the alkoxy oxygen also donates two electrons to form a second covalent bond thus resulting in the five membered chelate ring. It also contains resonance electrons in the benzene ring which contributes to the stabilization of the five membered chelate ring. Metal chelates have a slight to neutral taste, and are absorbed more readily than inorganic mineral salts. Hence, the present study deals with the formation of herbomineral complex of vanillin with zinc to obtain improved anticancer activity [3].
Materials and Methods
Experimental section
Vanillin (99%) was gifted by K Patel Phytoextract Pvt Ltd, Mumbai, doxorubicin, ethanol (95%), methanol and 0.1N HCL (SD Fine Chem Pvt Ltd), zinc acetate dihydrate [Zn(CH3COO)2.2H2O], murexide, filtered double distilled water (0.22 μm membrane filter). All other chemicals and reagents were either spectroscopic or analytical grade [4].
Formation of vanillin Zn complex
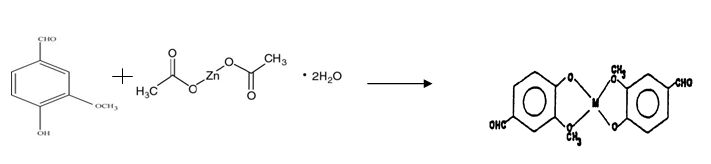
Vanillin (0.5 g) was dissolved in 50 ml hot boiling 95% ethanol. To this was added 2 g of zinc acetate dehydrate [Zn(CH3COO)2.2H2O] dissolved in 50 ml warm distilled water. The solution was allowed to stand overnight. The complex precipitated out as rhombic shaped crystals (Figure 1).
A complex thus formed was centrifuged, washed several times with distilled water and 95 percent ethanol to remove any unreacted starting material, dried and collected. Yield obtained was 0.66 g.
Evaluation
Physical properties: The active phytoconstituent (vanillin) and the Vanillin-Zn complex were theoretically and practically observed to compare the physical properties like taste and melting point [5].
Metal chelating activity assay
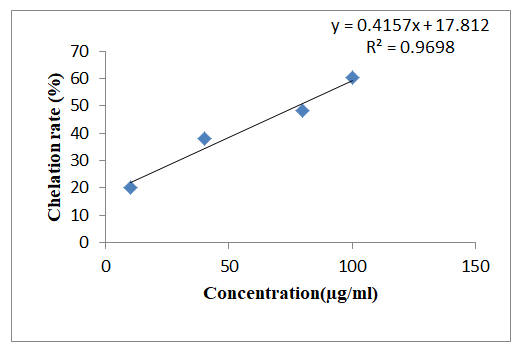
Vanillin solution (1 mg/mL) was prepared and further diluted using methanol to obtain 10 μg/ml, 40 μg/ml, 80 μg/ml and 100 μg/ml respectively. The chelating activity of vanillin for zinc ions was measured. 0.5 ml of diluted solutions were added in labeled test tubes and kept in a boiling water bath. To this was added 1.6 mL of deionized water and warm 0.1 mL of zinc acetate dehydrate (8 mM). After the solution was cooled, 0.1 mL murexide (5 mM) was added. Murexide reacted with the divalent zinc to form water soluble stable magenta complex. After 10 min at room temperature, the absorbance of the zinc complex was measured at 562 nm. The chelation rate of vanillin for Zn2+ was calculated as follows [6].
Chelation rate (%)=(A0-A1)/A0 × 100
Where A0 was the absorbance of the control (blank, without standard vanillin) and A1 was the absorbance in the presence of the vanillin.
UV-visible spectroscopy
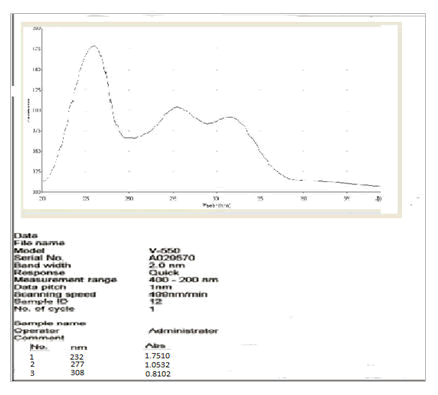
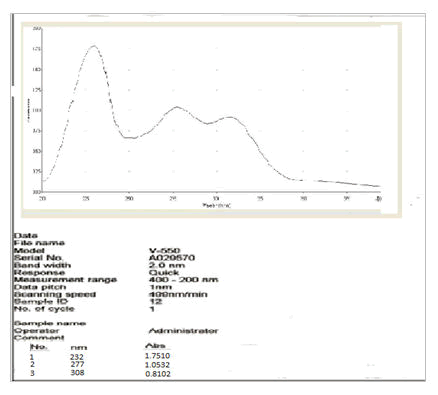
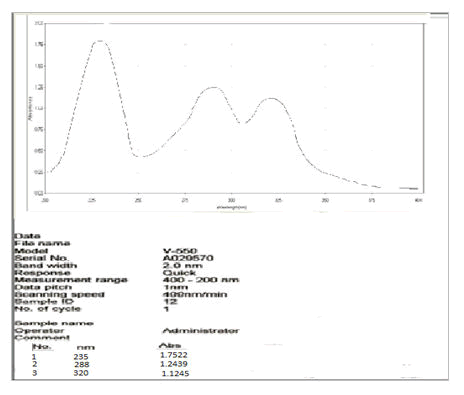
Vanillin (10 mg) and vanillin zinc complex (10 mg) were weighed and dissolved in 10 mL methanol respectively to obtain a stock solution of 1 mg/mL. This stock solution was further diluted to get a concentration of 10 μg/ml. Ultraviolet spectra were efficiently recorded on JASCO V-550 UV-Vis spectrophotometer [7].
Inductively coupled plasma atomic emission spectroscopy
ICP-AES is an emission spectrophotometric technique which utilizes plasma as the source of atomization and excitation. The excited electrons emit energy at a given wavelength as they return to ground state after excitation by high temperature argon plasma. The fundamental characteristic of this process is that each element emits energy at specific wavelengths peculiar to its atomic character. The energy transfer for electrons when they fall back to ground state is unique to each element as it depends upon the electronic configuration of the orbital. The energy transfer is inversely proportional to the wavelength of electromagnetic radiation, E=hc/λ (where h is planck's constant, c is the velocity of light and λ is wavelength), and hence the wavelength of light emitted is also unique. Each element emits energy at multiple wavelengths, in the ICP-AES technique it is most common to select a single wavelength for a given element. The intensity of the energy emitted at the chosen wavelength is proportional to the concentration of that element in the sample being analyzed. Inductively coupled plasma atomic emission spectral analysis was carried out by IIT Bombay. Here, ICP-AES was used for calculating the content of zinc in the complex [8].
Sample preparation
20 mg of standard vanillin and vanillin zinc complex was solubilized with 20 ml of conc. HCL to obtain a clear solution
• Sample I-standard vanillin
• Sample II-vanillin-zinc complex
Nuclear magnetic resonance
Nuclear Magnetic Resonance (NMR) spectroscopy is the most powerful tool available for determining the structure of organic compounds. This technique relies on the ability of atomic nuclei to behave like a small magnet and align them with an external magnetic field. When irradiated with a radio frequency signal the nuclei in a molecule can change from being aligned with the magnetic field to being opposed to it. Therefore, it is called nuclear for the instrument works on stimulating the nuclei of the atoms to absorb radio waves. The energy frequency at which this occurs can be measured and is displayed as an NMR spectrum [9].
Mass spectroscopy
A mass spectrum is obtained by converting the compounds of a sample into rapidly moving ions (usually positive) and resolving them on the basis of their mass to charge ratio. The utility of mass spectrometry arises from the fact that the ionization process generally produces a family of positive e particles whose mass distribution is characteristic of the parent species. As a consequence, a mass spectrum provides information that is useful for elucidating chemical structures. Mass spectral data are easier to interpret than infrared and NMR spectra in some respects, since they provide information in terms of molecular mass of the structural components of a sample in addition an accurate measure of the molecular weight of the analyte can usually be obtained from the data. Mass spectra was recorded using methanol as solvent on JT baker, waters xevo Tqs triple quadpole LC and MS at a flow rate 10 μl/min and ionization type electrospray [10].
Differential scanning calorimetry
Thermal analysis was conducted using Pyris 6 differential scanning calorimeter (PerkinElmer, Netherlands). Powdered vanillin and vanillin-Zn complex were accurately weighed (5 mg) in aluminum pans, sealed and subjected to DSC under nitrogen flow (20 mL/min) using a Perkin Elmer Pyris 6 DSC thermal analysis instrument. Thermograms were recorded by heating samples from 30°C to 300°C at a heating rate of 10°C min-1 with empty aluminum pan as the reference [11].
In vitro anticancer activity
In vitro testing of anticancer activity was carried out in collaboration with Tata memorial centre (Advanced centre for treatment, research and education in cancer).
Sulforhodamine assay
The sulforhodamine assay is a rapid, sensitive, and inexpensive method for measuring the cellular protein content of adherent and suspension cultures in 96-well microtiter plates. The method is suitable for ordinary laboratory purposes and for very large scale applications [12]. The cell lines were grown in RPMI 1640 medium containing 10% fetal bovine serum and 2 mM L-glutamine. For present screening experiment, cells were inoculated into 96 well microtiter plates in 100 μL at plating densities as shown in the study details above, depending on the doubling time of individual cell lines. After cell innoculation, the microtiter plates were incubated at 37°C, 5% CO2, 95% air and 100% relative humidity for 24 h prior to addition of experimental drugs [13]. After 24 h, one 96 well plate containing 5 × 1 03 cells/well was fixed in situ with TCA, to represent a measurement of the cell population at the time of drug addition (Tz). Experimental drugs were initially solubilized in ethanol at 100 mg/ml and diluted to 1 mg/ml using water and stored frozen prior to use. At the time of drug addition, an aliquot of frozen concentrate (1 mg/ml) was thawed and diluted to 100 μg/ml, 200 μg/ml, 400 μg/ml and 800 μg/ml with complete medium containing test article. Aliquots of 10 μl of these different drug dilutions were added to the appropriate microtiter wells already containing 90 μl of medium, resulting in the required final drug concentrations i.e., 10 μg/ml, 20 μg/ml, 40 μg/ml, 80 μg/ml [14].
End point measurement
After compound addition, plates were incubated at standard conditions for 48 hours and assay was terminated by the addition of cold TCA. Cells were fixed in situ by the gentle addition of 50 μl of cold 30% (w/v) TCA (final concentration, 10% TCA) and incubated for 60 minutes at 4°C. The supernatant was discarded; the plates were washed five times with tap water and air dried. Sulforhodamine B (SRB) solution (50 μl) at 0.4% (w/v) in 1% acetic acid was added to each of the wells, and plates were incubated for 20 minutes at room temperature [15]. After staining, unbound dye was recovered and the residual dye was removed by washing five times with 1% acetic acid. The plates were air dried. Bound stain was subsequently eluted with 10 mM tris base, and the absorbance was read on a plate reader at a wavelength of 540 nm with 690 nm reference wavelength. Percent growth was calculated on a plate by plate basis for test wells relative to control wells.
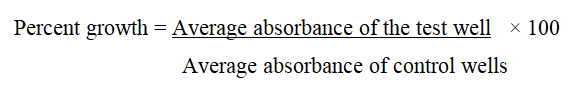
Using the six absorbance measurements Time zero (Tz), Control growth (C), and test growth in the presence of drug at the four concentration levels (Ti), the percentage growth was calculated at each of the drug concentration levels. Percentage growth inhibition was calculated as.
[(Ti-Tz)/(C-Tz)] × 100 for concentrations for which Ti ≥ Tz(Ti-Tz) positive or zero.
[(Ti-Tz)/Tz] × 100 for concentrations for which Ti<Tz (Ti-Tz) negative
The dose response parameters were calculated for each test article. Growth inhibition of 50% (GI50) was calculated from [(Ti-Tz)/(C-Tz)] × 100=50, which is the drug concentration resulting in a 50% reduction in the net protein increase (as measured by SRB staining) in control cells during the drug incubation. The drug concentration resulting in Total Growth Inhibition (TGI) was calculated from Ti=Tz. The LC50 (concentration of drug resulting in a 50% reduction in the measured protein at the end of the drug treatment as compared to that at the beginning) indicating a net loss of cells following treatment is calculated from [(Ti-Tz)/Tz] × 100=50. Values were calculated for each of these three parameters, the level of activity was reached; however, if the effect was not reached or was exceeded, the values for that parameter were expressed as greater or less than the maximum or minimum concentration tested.
Results and Discussion
Physical properties
Complex formation was confirmed by comparing the practical and theoretical values (Table 1).
|
Taste |
Melting point (°C) |
|||
|---|---|---|---|---|
| Sample | Theoretical | Observed | Theoretical | Observed |
| Vanillin | Sweet | Sweet | 82 | 81 |
| Vanillin zinc complex | Neutral | Neutral | >100 | 153 |
Table 1. Physical properties of vanillin and vanillin-Zn complex.
Metal chelating activity assay
Chelation rate (%) evaluation by metal chelating activity assay showed increase in the rate of chelation with increase in vanillin concentration. Chelating activity of vanillin is mainly attributed to the presence of hydroxyl and methoxy group attached to the benzene ring (Figure 2).
UV-vis spectroscopy
UV spectral analysis of the vanillin-zinc complex showed a bathochromic shift as compared to the parent drug which may be because of the chelate formation (Figures 3 and 4).
Inductively coupled plasma atomic emission spectroscopy
ICP-AES was carried out to determine the percentage of zinc in the synthesized complex. No significant difference was observed between the practical and theoretical value. Theoretical (%Zn) was calculated by considering that the ligand to zinc ratio of 2:1 to form a complex (Table 2).
| Concentration of zinc (ppm) | % of zinc | |
|---|---|---|
| Theoretical | Practical | |
| 180.2 | 17.55 | 18.2 |
Table 2. Concentration of zinc in vanillin-Zn complex.
Nuclear magnetic resonance spectroscopy
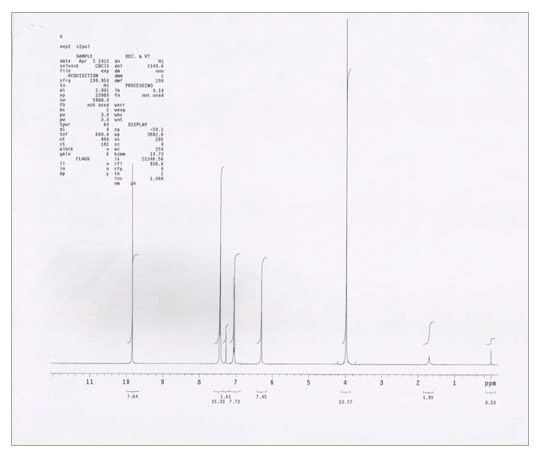
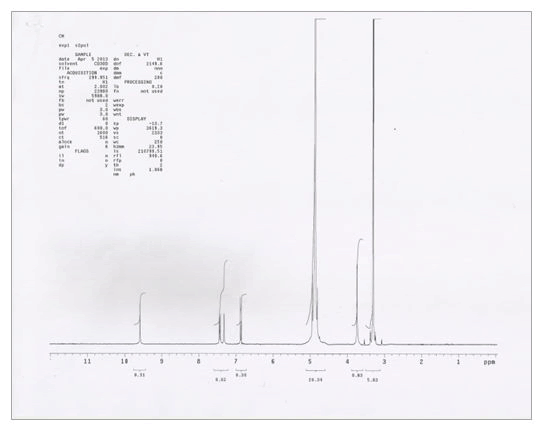
NMR spectra of vanillin and vanillin-zinc complex shows that the prominent peak attributable to -OH i.e., 6.29 s present in vanillin disappeared in the herbomineral complex, thus indicating that Zn+2 ion is bonding with hydroxyl oxygen of vanillin. Moreover, 3.97 s attributable to -OCH3 group was shifted to 3.74 s in the herbomineral complex indicating its involvement in complex formation (Table 3, Figures 5 and 6).
| Sample | Chemical shift (ppm) | Multiplicity | Group |
|---|---|---|---|
| Standard vanillin | 7.42 | Doublet | -H |
| 7.06 | Doublet | -H | |
| 7.26 | Singlet | -H | |
| 3.97 | Singlet | 0 | |
| 6.29 | Broad singlet | -OH | |
| 9.83 | Singlet | -CHO | |
| 7.42 | Double doublet | -H | |
| Vanillin-Zn complex | 6.88 | Doublet | -H |
| 7.32 | Singlet | -H | |
| 3.74 | Singlet | 0 | |
| 9.58 | Singlet | -CHO | |
| _ | Disappear | -OH |
Table 3. Analysis of NMR spectra of vanillin-Zn complex.
Mass spectroscopy
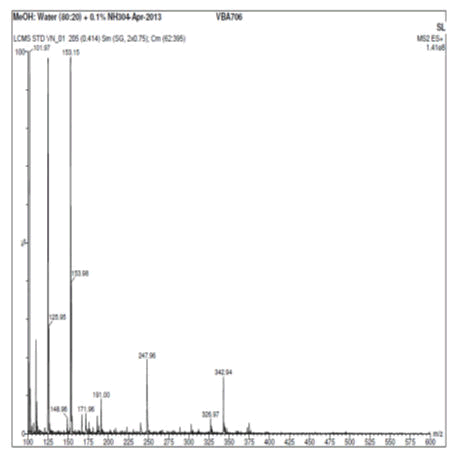
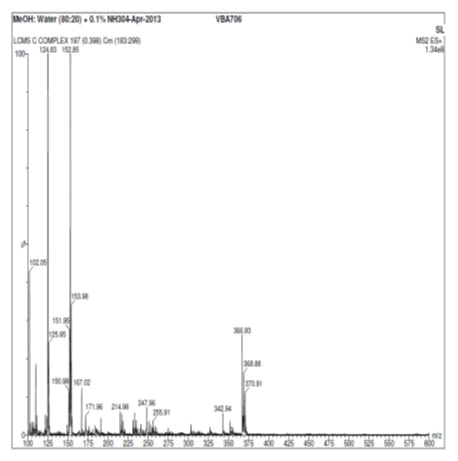
Vanillin and its zinc complex were subjected to mass spectral analysis, which confirmed the molecular mass of vanillin and vanillin zinc complex as 153.15 and 368.88 respectively. The theoretical mass of the chelate was calculated that well correlated with two vanillin molecules binding with one molecule of zinc to form a chelate. The mass spectral studies thus confirmed the aforesaid ratio of vanillin and zinc in the herbomineral complex (Figures 7 and 8).
Differential scanning calorimetry
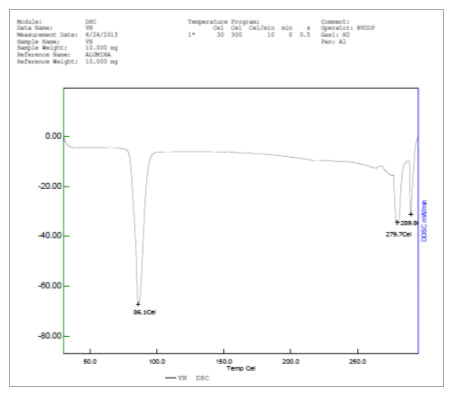
The thermal profile of vanillin and vanillin zinc complex carried by differential scanning calorimetry at scan rate 10°C/min revealed that vanillin showed a sharp melting endotherm at 86.1°C whereas vanillin-Zn complex showed the melting endotherm at 182.3°C This shows that formation of herbomineral complex increases the stability of the drug towards thermal degradation (Figures 9 and 10).
In vitro anticancer activity
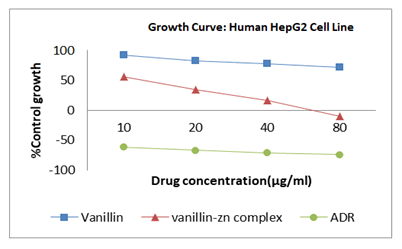
Vanillin and vanillin-zinc complex were evaluated and compared for anticancer activity. Formation of herbomineral complex showed significant increase in anticancer activity as compared to vanillin. Vanillin gets rapidly decomposed in the upper digestive tract hence it shows poor bioavailability. This prompted us to form the herbomineral complex of vanillin with zinc (Figures 11 and 12) (Table 4).
| HEPG2 | Drug concentrations (µg/ml) | ||
|---|---|---|---|
| LC50 | TGI | GI50 | |
| A | >80 | >80 | >80 |
| B | 52.5 | 31.4 | 11.1 |
| ADR | 21.5 | <10 | <10 |
Note: A-Vanillin, B-Vanillin-Zn complex, ADR-adriamycin(doxorubicin)
Table 4. Effect of vanillin and vanillin-Zn complex on human hepatic G2 cell line.
The present study implicits that the vanillin-Zn herbomineral complex (GI50 11.1 μg/ml) possess promising anticancer activity against the HepG2 cell line. Herbomineral complex showed improved anticancer activity, this may be due to the fact that Zn is excreted mainly in feces and only traces found in the urine, since kidney has little role in regulating body Zn content. Endogenous Zn reabsorbed in ileum and colon creating enterohepatic circulation of Zn. The peak plasma concentration occurs in approximately 2 hours. From the above kinetics of Zn, it was shown that, herbomineral complex formed by using zinc, also get reabsorbed in kidney, thus gives anticancer activity for longer period of time. Hence, the greater anticancer potential of vanillin-Zn herbomineral complex compared with is attributed to its improved bioavailability. However, the in depth mechanism of anticancer activity needs to be further investigated.
Conclusion
The unique properties of herbomineral complexes tend to offer advantages in the discovery and development of new drugs. Additionally, the effects of mineral such as zinc can be highly specific and can be modulated by recruiting cellular processes. These can be useful probes of cellular function. Understanding these interactions could lead the way towards rational design of herbomineral complex formulation and implementation of new co therapies.
Acknowledgement
Author is thankful to university grants commission special assistance program (UGC-SAP) for providing research fellowship.
References
- Turrini E, Calcabrini C, Tacchini M, et al. In vitro study of the cytotoxic cytostatic and antigenotoxic profile of Hemidesmus indicus (L.) R. Br. (Apocynaceae) crude drug extract on T lymphoblastic cells. Toxins. 2018;10(2):69-70.
[Crossref] [Google Scholar] [PubMed]
- Cosme P, Rodríguez AB, Espino J, et al. Plant phenolics: Bioavailability as a key determinant of their potential health promoting applications. Antioxidants. 2020;9(12):1263.
[Crossref] [Google Scholar] [PubMed]
- Haas KL, Franz KJ. Application of metal coordination chemistry to explore and manipulate cell biology. Chem Rev. 2009;109(10):4921-60.
[Crossref] [Google Scholar] [PubMed]
- Guengerich FP, Yoshimoto FK. Formation and cleavage of C-C bonds by enzymatic oxidation-reduction reactions. Chem Rev. 2018;118(14):6573-655.
[Crossref] [Google Scholar] [PubMed]
- Badgujar NV, Mistry KN, Chudasama PN, et al. In vitro antioxidant and cytotoxic effects of methanol extracts of Vitex negundo, Lantana camara, Bauhinia variegata and Bauhinia racemosa on human cancer cell lines. Indian J Pharma Sci. 2017;79(3):431-7.
- Ojeda CB, Rojas FS. Recent applications in derivative ultraviolet/visible absorption spectrophotometry: A review. Microchem J. 2013;106:1-6.
- Qian J, Zhou CH, Qian Z, et al. Development of a K562 cell based assay for screening anticancer agents. Acta Pharmacol Sin. 2001;22(9):821-6.
[Google Scholar] [PubMed]
- Orellana EA, Kasinski AL. Sulforhodamine B (SRB) assay in cell culture to investigate cell proliferation. Bio Protoc. 2016;6(21):1983-84.
[Crossref] [Google Scholar] [PubMed]
- Samoszuk M, Tan J, Chorn G. The chalcone butein from Rhus verniciflua stokes inhibits clonogenic growth of human breast cancer cells co-cultured with fibroblasts. BMC Complement Altern Med. 2005;5:1-5.
[Crossref] [Google Scholar] [PubMed]
- Liu Q, Jackstell R, Beller M. Oxidative catalytic coupling reactions: Selective formation of C-C and C-X bonds using radical processes. Angew Chem Int Ed Engl. 2013;52(52):13871-73.
[Crossref] [Google Scholar] [PubMed]
- Kareem A, Arshad M, Nami SA, et al. Herbo-mineral based Schiff base ligand and its metal complexes: Synthesis, characterization, catalytic potential and biological applications. J Photochem Photobiol Biol. 2016;160:163-71.
[Crossref] [Google Scholar] [PubMed]
- Watak S, Patil SS. Evaluation and comparison of antioxidant activity of herbomineral complex. Res J Pharmaco Phytochem. 2012;4(3):171-7.
- Zhang Q, Zhang Q, Li ZZ, et al. Copper (II) complexes modified with water-soluble porphyrin and various small molecules ligand for DNA binding and potential selectivity antitumor agents. Dyes Pigm. 2020;173:107923.
- Cai L, Zhu P, Huan F, et al. Toxicity-attenuated mesoporous silica Schiff-base bonded anticancer drug complexes for chemotherapy of drug resistant cancer. Collo Surf Biointer. 2021;205:111839.
[Crossref] [Google Scholar] [PubMed]
- Sulaiman MK, Lakshmanan J. Systemic and anticancer potential of adaptogenic constituents isolated from traditional herbs-a mini-review. Anti Cancer Agen Med Chem. 2022;22(16):2811-21.
[Crossref] [Google Scholar] [PubMed]