Review Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2022) Volume 12, Issue 92
Corona Virus: A detailed review of COVID-19.
Jyoti Devi1, Ashish Kumar2*, Ajay Kumar2
1Research Scholar of Pharmaceutics, Himalayan institute of Pharmacy, Kala Amb, District Sirmour, Kala Amb, Himachal Pradesh, India
2Department of Pharmaceutics, School of Pharmacy, Arni University, Kathgargh District, Kangra, Himachal Pradesh, India
- Corresponding Author:
- Ashish Kumar
Assistant professor of Pharmaceutics
School of Pharmacy, Arni University
Kathgarh, Indora, Distt. Kangra
Himachal Pradesh, India
E-mail: abukumar1997@gmail.com
Received: 26-July-2022, Manuscript No. AABPS-22-70287; Editor assigned: 29-July-2022, PreQC No. AABPS-22-70287(PQ); Reviewed: 12-Aug-2022, QC No. AABPS-22-70287; Revised: 16-Aug-2022, Manuscript No. AABPS-22-70287(R); Published: 23-Aug-2022, DOI:10.35841/2249-622X.92.140
Citation: Kumar A. Corona Virus: A detailed review of COVID-19. Asian J Biomed Pharmaceut Sci. 2022;12(92):140
Abstract
In late December 2019, the health officials in China reported of a mysterious pneumonia with no known Etiology. Prompt genome analysis revealed that the disease was the cause of a novel coronavirus. As the outbreak continued to spread out at a remarkable pace, the World Health Organization (WHO) had to declare it a pandemic on March 11, 2020. SARS-CoV-2 is the seventh coronavirus to infect humans and the causative agent for COVID-19. Previously, two coronavirus epidemics raised international concern, SARS in 2002–2003 and MERS in 2012. As for the new virus, it is highly infectious and has already killed over 200,000 people with an estimated sCFR (symptomatic case fatality risk) of 1.4% (0.9–2.1%) (Wu JT, Leung K, Bushman M, 2020).
Keywords
Corona Virus, SARS, WHO, Nucleic acid amplification test, Reverse transcription polymerase chain reaction.
Introduction
By comparison, fatality due to SARS was roughly 10%, whereas, for MERS, it was as high as 35%, making it one of the deadliest human [1]. However, SARS-CoV-2 has been shown to have much higher human-to-human transmissibility [2]. With the ability to infect people through asymptomatic carriers, it can remain unnoticed and quickly disseminate itself, making the disease containment a confounding public health challenge [3]. WHO recommends nucleic acid amplification test (NAAT) based reverse transcription polymerase chain reaction (RT-PCR) as the primary testing method because of its accuracy and hence it remains the gold standard for COVID-19 detection (WHO, Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases. Interim Guidance, WHO, Geneva, Switzerland, 2020). However, the technique requires laboratory settings as well as skilled personnel to conduct the test with precision. To scale up the number of tests performed per day, the need for the development of an accurate point-of-care test is of paramount importance. Recently, some antibody-based serological studies provided insights that the number of people having COVID-19 infection could be much higher than what was previously thought.
With overloaded healthcare and an increasing number of infections among the medical personnel, the ultimate way out remains to be in the discovery of an effective vaccine. However, the discovery and development of vaccine or drugs is a lengthy process, and it generally takes around a decade to pass through the entire course. So, repurposing of existing drugs to treat COVID-19 appears as a logical scientific approach. So far, the choices are limited for the treatment of COVID-19 by a lack of specific drugs. From a variety of existing antiviral medications, repurposing the appropriate drug remains as a challenge to overcome. Small-scale studies reported a few drugs to be effective, but later proved to bring no significant difference in clinical [4]. High throughput virtual screening and in vitro studies are underway to look for scopes in both the development and repurpose able options of antiviral drugs.
As the virus is moving in a pandemic speed, various control measures have been considered by different regions. Revising the decisions and their respective outcomes can further resolve the challenges in disease containment. In this review, we aimed at summarizing the current literatures to draw a compendium of understanding on the scopes of diagnostics tools, treatments, vaccines, and control measures for COVID-19 [5].
Viral morphology
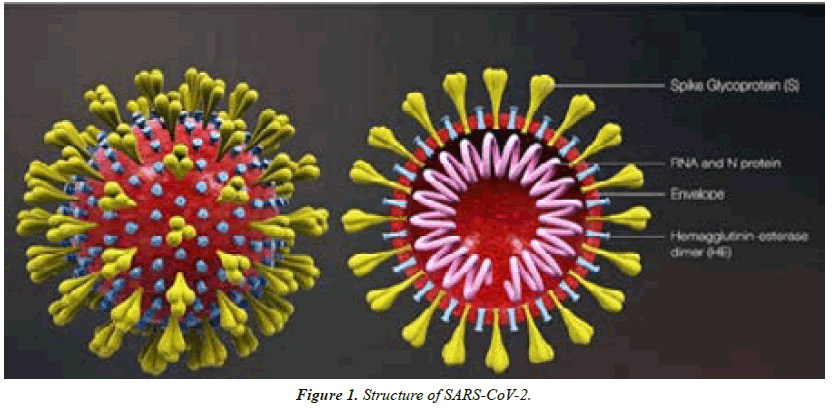
Coronaviruses are enveloped, positive single-stranded RNAs with the largest known RNA genome ranging from 26 to 32 kilo bases in length. They are spherical Virions with a core shell awnd a surface that resembles a solar corona based on its surface protein projections, hence their name (Latin: corona = crown). There are four main subfamilies; alpha-, beta-, gamma- and delta- coronaviruses. Alpha- and betacoronaviruses originate from mammals, mainly bats, and are thought to cause more severe and fatal diseases in humans, while gamma- and delta-viruses mainly originate from birds and pigs and are thought to cause asymptomatic or mild disease in humans [5].
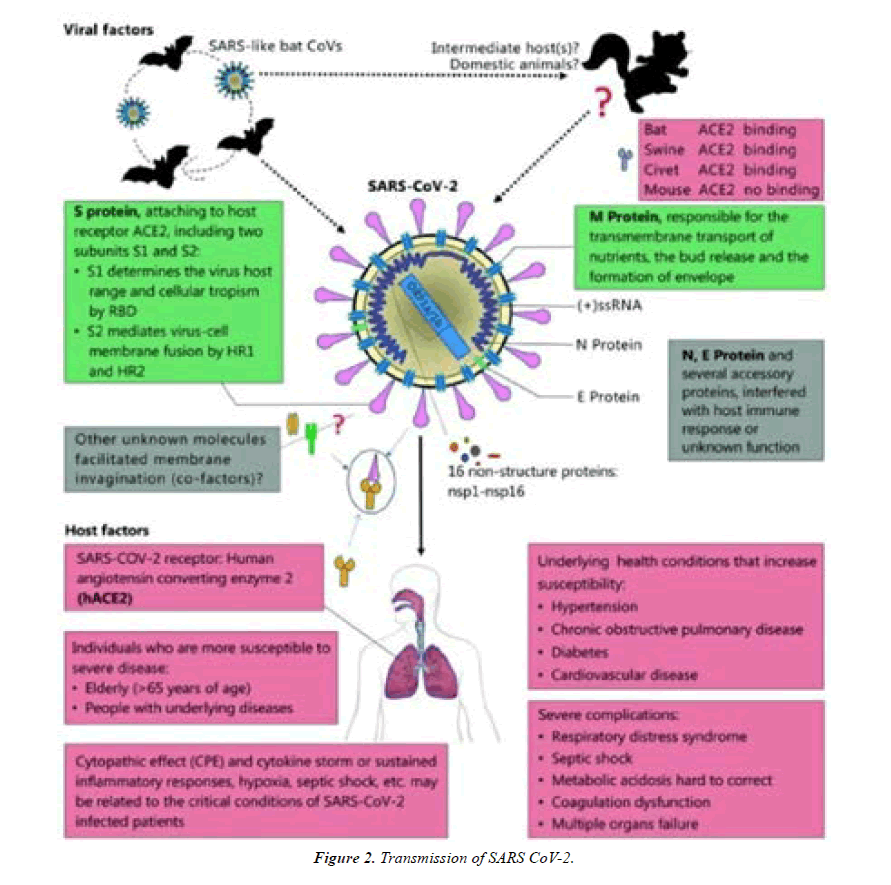
SARS-CoV-2 belongs to the beta-coronavirus group, which also includes MERS-CoV and SARS-CoV. The latter shares ~75–80% of its viral genome with SARS-CoV2. Betacoronaviruses have three important envelope proteins: Spike (S) protein, Membrane (M) protein, and Envelope protein. S protein mediates viral attachment to the cell membrane receptor, membrane fusion, and ultimately viral entry into the host cell. M protein, the most abundant membrane protein, together with E protein is responsible for the coronavirus membrane protein structure. Another component of the beta-coronavirus is the N protein, which is the protein component of the helical nucleocapsid that includes the genome RNA [6] (Figure 1).
Modes of transmission
This section briefly describes possible modes of transmission for SARS-CoV-2, including contact, droplet, airborne, fomite, fecal-oral, blood borne, mother-to-child, and animal-tohuman transmission. Infection with SARS-CoV-2 primarily causes respiratory illness ranging from mild disease to severe disease and death, and some people infected with the virus never develop symptoms [7].
Contact and droplet transmission
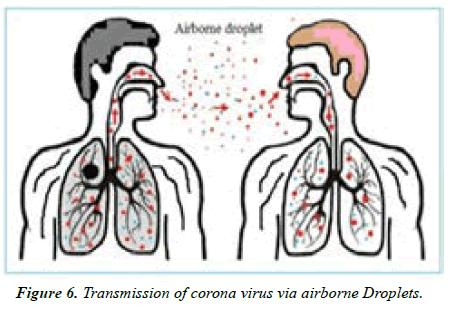
Transmission of SARS-CoV-2 can occur through direct, indirect, or close contact with infected people through infected secretions such as saliva and respiratory secretions or their respiratory droplets, which are expelled when an infected person coughs, sneezes, talks or sings. Respiratory droplets are >5-10 μm in diameter whereas droplets <5μm in diameter are referred to as droplet nuclei or aerosols. Respiratory droplet transmission can occur when a person is in close contact (within 1 meter) with an infected person who has respiratory symptoms (e.g. coughing or sneezing) or who is talking or singing; in these circumstances, respiratory droplets that include virus can reach the mouth, nose or eyes of a susceptible person and can result in infection. Indirect contact transmission involving contact of a susceptible host with an object or surface (fomite transmission) may also be possible [7] (Figure 2).
Airborne transmission
Airborne transmission is defined as the spread of an infectious agent caused by the dissemination of droplet nuclei (aerosols) that remain infectious when suspended in air over long distances and time. Airborne transmission of SARS-CoV-2 can occur during medical procedures that generate aerosols (“aerosol generating procedures”). WHO, together with the scientific community, has been actively discussing and evaluating whether SARS-CoV-2 may also spread through aerosols in the absence of aerosol generating procedures, particularly in indoor settings with poor ventilation?
Some outbreak reports related to indoor crowded spaces have suggested the possibility of aerosol transmission, combined with droplet transmission, for example, during choir practice, in restaurants or in fitness classes. In these events, short-range aerosol transmission, particularly in specific indoor locations, such as crowded and inadequately ventilated spaces over a prolonged period of time with infected persons cannot be ruled out. However, the detailed investigations of these clusters suggest that droplet and fomite transmission could also explain human-to-human transmission within these clusters. Further, the close contact environments of these clusters may have facilitated transmission from a small number of cases to many other people (e.g., super spreading event), especially if hand hygiene was not performed and masks were not used when physical distancing was not maintained [8].
Fomite transmission
Respiratory secretions or droplets expelled by infected individuals can contaminate surfaces and objects, creating fomites (contaminated surfaces). Viable SARS-CoV-2 virus and/or RNA detected by RT-PCR can be found on those surfaces for periods ranging from hours to days, depending on the ambient environment (including temperature and humidity) and the type of surface, in particular at high concentration in health care facilities where COVID-19 patients were being treated. Therefore, transmission may also occur indirectly through touching surfaces in the immediate environment or objects contaminated with virus from an infected person (e.g. stethoscope or thermometer), followed by touching the mouth, nose, maintained [9].
Other modes of transmission
Evidence to date shows that SARS-CoV-2 is most closely related to known beta corona viruses in bats; the role of an intermediate host in facilitating transmission in the earliest known human cases remains unclear. In addition to investigations on the possible intermediate host(s) of SARSCoV- 2, there are also a number of studies underway to better understand susceptibility of SARS-CoV-2 in different animal species. Current evidence suggests that humans infected with SARS-CoV-2 can infect other mammals, including dogs, cats, and farmed mink. However, it remains unclear if these infected mammals pose a significant risk for transmission to humans [10].
COVID-19 'super-spreading' events play outsized role in overall disease transmission
There have been many documented cases of Covid-19 “superspreading” events, in which one person infected with the SARSCoV- 2 virus infects many other people. But how much of a role do these events play in the overall spread of the disease? A new study from MIT suggests that they have a much larger impact than expected. The study of about 60 super-spreading events shows that events where one person infects more than six other people are much more common than would be expected if the range of transmission rates followed statistical distributions commonly used in epidemiology. Based on their findings, the researchers also developed a mathematical model of COVID-19 transmission, which they used to show that limiting gatherings to 10 or fewer people could significantly reduce the number of super-spreading events and lower the overall number of infections [11].
Clinical Symptoms
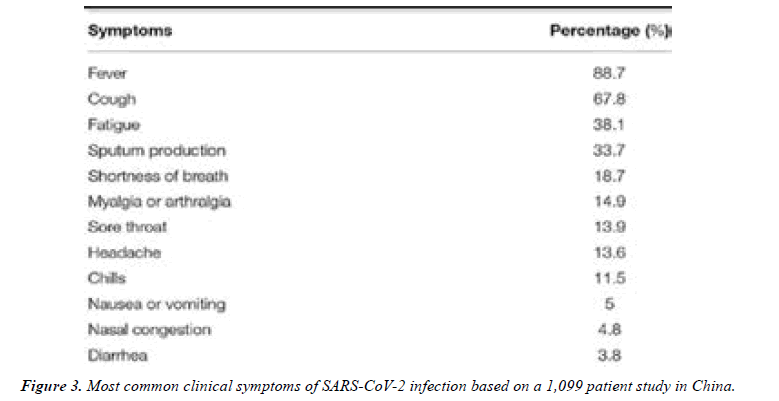
Anosmia and dysgeusia have also been reported in patients with SARS-CoV-2 infection. A cross-sectional survey study found that these symptoms were frequently reported in patients infected with SARS-CoV-2 and, in most cases, preceded the onset of other symptoms. Asymptomatic infection has also been discussed in the literature; however, the frequency remains unclear. A study of 55 asymptomatic carriers with confirmed SARS-CoV-2 infection on admission found that the majority of these patients ended up having mild symptoms and a mild disease course [12]. While asymptomatic infection was rare and was mainly in young patients between 18 and 29 years of age. Another study involving 634 patients infected with COVID-19 on a cruise ship in Japan found that 17.9% were asymptomatic [13] (Figure 3).
Pathogenesis
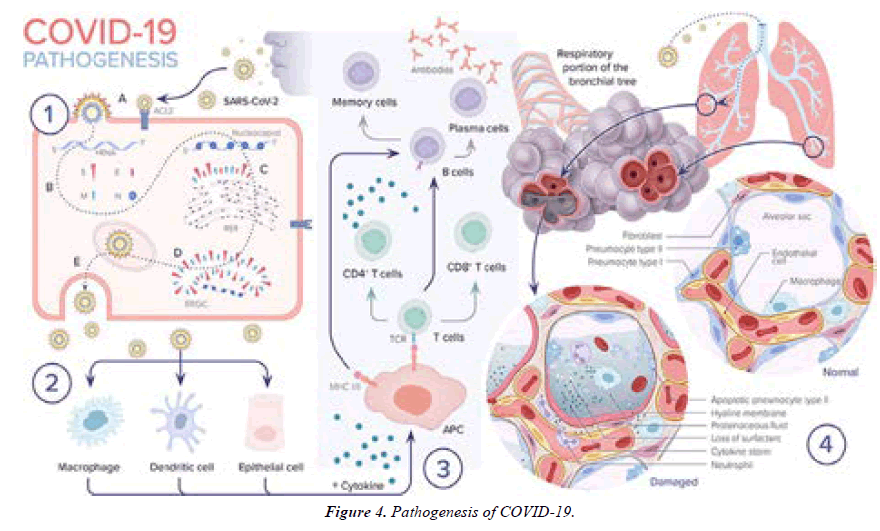
1. A SARS-CoV-2 enters the epithelial cell either via endocytosis or by membrane fusion through binding to ACE2 receptor and releasing its RNA into the cytoplasm. B. Viral RNA uses the cell’s machinery to translate its viral non-structural and structural proteins and replicate its RNA. C. Viral structural proteins S, E, and M assemble in the rough endoplasmic reticulum (RER). D. Viral structures and nucleocapsid subsequently assemble in the endoplasmic reticulum Golgi intermediate (ERGIC). E. New virion packed in Golgi vesicles fuse with the plasma membrane and get released via exocytosis.
2. SARS-CoV-2 infection induces inflammatory factors that lead to activation of macrophages and dendritic cells.
3. Antigen presentation of SARS-CoV-2 via major histocompatibility complexes I and II (MHC I and II) stimulates humoral and cellular immunity resulting in cytokine and antibody production.
4. In severe COVID-19 cases, the virus reaches the lower respiratory tract and infects type II pneumocytes leading to apoptosis and loss of surfactant. The influx of macrophages and neutrophils induces a cytokine storm. Leaky capillaries lead to alveolar edema. Hyaline membrane is formed. All of these pathological changes result in alveolar damage and collapse, impairing gas exchange (Figure 4).
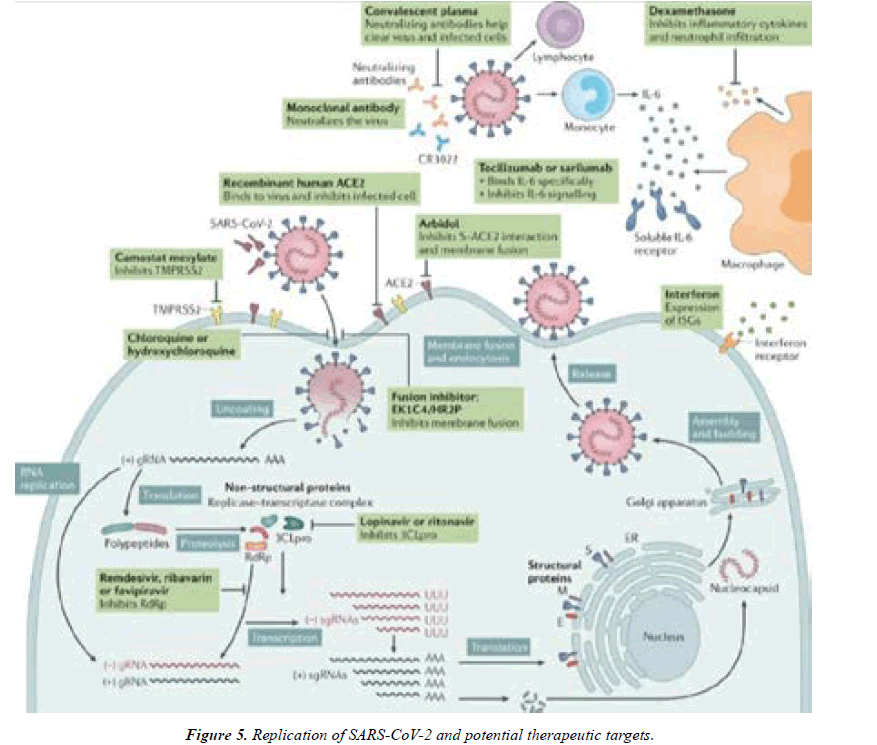
SARS-CoV-2 replication and potential therapeutic targets
Potential antivirals target the different steps of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication, ranging from receptor binding, entry and fusion to replication. Furthermore, immunoglobulin-based and immunomodulatory drugs are potential therapeutics as well. Note that robust data on clinical efficacy are lacking for most of these treatments so far. 3CLpro, 3C-like protease; ACE2, angiotensin-converting enzyme 2; CR3022, a SARS-CoVspecific human monoclonal antibody; E, envelope protein; EK1C4, lipo peptide derived from EK1 which is a pancoronavirus fusion inhibitor targeting the HR1 domain of the spike protein; ER, endoplasmic reticulum; gRNA, genomic RNA; HR2P, heptad repeat 2-derived peptides of SARSCoV- 2 spike protein; IL-6, interleukin-6; ISG, interferonstimulated gene; M, membrane protein; RdRp, RNAdependent RNA polymerases; sgRNA, sub genomic RNA; S, spike protein; TMPRSS2, transmembrane protease serine protease 2 [14] (Figure 5).
Diagnosis
The Real Time Reverse Transcriptase Polymerase Chain Reaction (rRT-PCR)
Currently, the targeted viral genes include N, E, S, and RdRP genes. An amplification of each of these genes can be accomplished by the supply of proper forward and reverse primers. One study reported that PCR amplification of the E and SARS coronavirus RdRp genes is 95% sensitive. The RT-PCR based diagnostic is highly sensitive, sequence specific, and useful in the early detection of COVID-19. Since the test accuracy varies depending on the disease stage and viral multiplication, the sensitivity can range from 71 to 98%, whereas the specificity is recorded to be 95% [15]. The RT-PCR has a few disadvantages such as the requirement of strict handling, time consumption, and a minimum initial concentration of RNA.
The lowest RNA concentration of SARS-CoV-2 detectable via RT-PCR is found to be 3.8–23 copies/ml. Heat treatment prior to RNA extraction is not recommended as studies suggest that thermal inactivation in samples with low viral loads could result in potential false-negative nucleic acid test. For areas afflicted with COVID-19, a negative PCR result does not imply the absence of the virus since a multitude of factors including viral mutation, PCR inhibition, improper handling of the sample, specimen collection time, low viral RNA, inappropriate shipment, or poor specimen quality can lead to a negative result in an infected individual.(False Negatives and Reinfections: The Challenges of SARS-CoV-2 RT-PCR Testing, American Society for Microbiology, Washington, DC, USA, 2020, https://asm.org/Articles/2020/April/False- Negatives-and-Reinfections-the-Challenges-of) [16].
Types of Specimen
Additionally, blood and stool specimen could be collected for clinical inspection. One study reported that the highest rates of positive were found from broncho alveolar lavage fluid (93%), followed by sputum (72%), nasal swab (63%), fibro broncho scope brush biopsy (46%), pharyngeal swabs (32%), feces (29%), and blood (1%). The test result for urine is usually negative, but the presence of SARS-CoV-2 in urine specimen was also described. However, autopsy materials such as lung tissue should be collected in case of deceased patients (WHO, Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases. Interim Guidance, WHO, Geneva, Switzerland, 2020). To retrospectively detect a case in a surviving patient, serological test is useful as antibodies for past infection are detected [17].
Development of Point-of-Care Tests
Some point-of-care molecular testing methods have already been approved by emergency use authorization (EUA). For instance, Accula SARS-CoV-2 testing is a qualitative visually read PCR method that provides results in 30 minutes; Abbott ID NOW COVID-19 test uses an isothermal nucleic acid amplification technology to target the RdRp gene, and the positive result can be obtained as quickly as 5 minutes (Accula SARS-CoV-2 Diagnostic Test for COVID-19—Mesa Biotech, Mesa Biotech, San Diego, CA, USA, 2020.
Immunological Test
Rapid tests targeting viral proteins: In addition, rapid test design for the detection of viral protein is also in progress. The viral nucleocapsid (N) and spike (S) are the main immunogenic proteins. While nucleocapsid protein is the most abundant and 90% similar to SARS-CoV, viral spike (S) protein is divergent and elicits strong immune response [17]. However, the S1 subunit of spike protein was found to be specific for SARS-CoV-2, but the S2 subunit of spike protein was conserved across coronaviruses. Since many people have antibodies to the four endemic human coronaviruses, targeting specific part of the spike protein could avoid cross-reactivity. Currently, virus culture for detection is not recommending as it is time-consuming and requires biosafety level 3 [18].
Serological tests targeting antibodies: Development of an accurate antibody testing is a major challenge, and as of now, hundreds of trials are ongoing. There are important considerations for antibody testing,
I. Timing of the test
II. Previous infection
III. Immune status of the individual, and
IV. Cross-reaction, which can alter the test result.
The ELISA based antibody test uses the technique of binding assay by way of a recombinant viral antigen that can bind to IgG. Other types of methods include a lateral flow chromatographic immunoassay that qualitatively assesses the presence of an analyte (e.g., antibody) from a patient’s whole blood, serum, or plasma specimen. The IgG antibodies bind to the recombinant SARS-CoV-2 antigen-coated gold nanoparticles (AuNP); the rabbit IgG gold-conjugates are used as control that binds to the anti-rabbit antibodies. The assay resembles a lateral flow pregnancy test but detects antibodies instead of a human glycoprotein. Recently, Abbott launched an IgG antibody test that received CE mark with its 99.6% specificity [19].
The Elecsys® AntiSARS-CoV-2 antibody test from Roche received FDA approval that employs in-solution doubleantigen sandwich format. The test can detect antibodies in human serum or plasma samples with specificity greater than 99.8% and sensitivity of 100% after 14 days of PCR confirmation [20].
CRISPR Technology
CRISPR gene editing tool has been employed to construct an accurate, faster, and simple-to-use SARS-CoV-2 detection test. DNA Endonuclease-Targeted CRISPR Trans Reporter (DETECTR) assay is based on CRISPR–Cas12 and it can distinguish SARS-CoV-2 with no cross-reactivity for related coronavirus strains using N gene gRNA within 40 minutes [21].
The result is visualized by using a FAM-biotin reporter molecule and lateral flow strips to capture labeled nucleic acids. Another CRISPR-based POC diagnostic is the SHERLOCK COVID-19 that incorporates a thermo stable Cas12b enzyme from Ali cyclo bacillus acidophilus. The test recently got FDA approval under emergency use authorizations [22].
The test can be conducted by extracting RNA from patient samples and can be read out in less than an hour using a dipstick, without requiring extensive instrumentation. In India, a different approach was taken to build a CRISPR-based tool. The FnCas9 Editor Linked Uniform Detection Assay (FELUDA) used a highly accurate enzymatic readout for detecting nucleotide sequences. The assay is quick to provide output and can be used in rapid diagnosis [23].
Imaging
Apart from detecting the presence of the virus, chest CT scan can demonstrate the disease status and severity. A recent study suggested that CT scan is more sensitive than the PCR procedure. During the early stage of pneumonia, there are multiple small patchy shadows seen with interstitial changes, which is unusual in the lung periphery. Some cases could develop bilateral multiple ground-glass opacity, infiltrating shadows, and pulmonary consolidation with infrequent pleural effusion [24].
Artificial Intelligence
Recently, artificial intelligence system based on deep convolutional neural network design was used to detect COVID-19 from chest radiography images. The tool provides quicker result and continues to get better with the addition of more data. Moreover, the machine learning approach shows potential to predict criticality in patients [25]. Some AI inspired mobile application-based tools are in development to preliminary detecting suspected COVID-19 patients.
Differential Diagnosis
Differential diagnosis is the process by which a single disease or condition is differentiated from those having similar clinical features. Patients with COVID-19 can have co infection or superimposed infection by other viruses or bacteria simultaneously. Differential diagnosis is therefore important to differentiate SARS-CoV-2 induced infection from other viral or bacterial and mycoplasma pneumonia. Since a plethora of clinical manifestations is observed in COVID-19, there is an increased chance for incorrect diagnosis. For instance, a COVID-19 patient in Thailand was found to present with fever and rash and was initially mistaken for dengue [26].
In regions with high burden of disease, differential diagnosis based on current symptoms, medical and epidemiological history and a set of physical examinations will help determine the proper etiology. In case of bacterial pneumonia, there is usually high fever and coughing with thick, blood-tinged mucus or yellowish-greenish sputum with pus. Mycoplasma pneumonia can also occur in any season, so blood culture or serum antibody determination is helpful for differential diagnosis [27].
Prevention
The WHO and other agencies such as the CDC have published protective measures to mitigate the spread of COVID?19.
Protective Measures for Citizens
However, unless one is sick, it is not recommended to use surgical masks or N95s, which are valuable resource for front-line healthcare workers (Recommendation Regarding the Use of Cloth Face Coverings, CDC, Atlanta, GA, USA, 2020, (https://www.cdc.gov/coronavirus/2019-ncov/preventgetting- sick/cloth-face-cover.html). COVID-19 is highly infectious, and contact transmission might occur due to touching the mouth, nose, or eyes with contaminated hands. On average, people tend to touch their faces every two and a half minutes, indicating how quickly the virus can make way into a human host [28]. With the ability of SARS-CoV-2 to stay viable on plastic and steel surfaces for up to 3 days, hand hygiene remains to be the effective way to avert the establishment of infection [29].
Protection for Healthcare Personnel
According to the European standard EN 149 + A1: 2009, there are three levels of precautions for healthcare workers: (i) contact, (ii) droplet, and (iii) airborne precaution (BS. EN 149:2001+A1:2009, Respiratory Protective Devices. Filtering Half Masks to Protect Against Particles. Requirements, Testing, Marking, BSI, Kolkata, India, 2001, [30] (Figures 6 and 7).
Medical personnel who work 2 meters away from the patients require contact protection and should wear gloves, mask, and apron. For those who work within 2 meters require droplet precaution and should also use fluid-resistant surgical mask and eyewear (e.g., goggles or a visor), whereas, for health professionals who perform aerosol generating procedures (AGP), they must be prioritized with supplies of gloves, fluidrepellent long sleeved gown, eye protection, and FFP2/3 mask topics [31].
FFP2, FFP3, and N95 are terms used to refer to high performance filtering masks made of a web of polypropylene microfibers and electrostatic charge. FFP2 and FFP3 can significantly reduce the concentration of hazardous substances up to 10- and 20-fold, respectively. It is important that healthcare workers have training prior to the use of PPE about its proper use and the way of disposal, as improper practice is associated with high rate of infection among the healthcare providers [32].
Lockdown
To contain the virus, a strict lockdown was imposed in Wuhan. Lockdown allows quick suppression of the number of infections facilitating time for the healthcare system to respond to the epidemic with planning and resource mobilization. One study showed that the infection number could be much higher in Mainland China without implementing social distancing. In two weeks, the R0 was reduced from 2.35 to 1.05 in Wuhan, as suggested by a modeling [33]. A similar effect was observed in the UK as the R0 was found to drop by 73% since the lockdown began (Impact of Physical Distance Measures on Transmission in the UK, CMMID Repository, 2020. In Singapore, the combination of school closure and social distancing in workplaces showed an efficacy of 99.3% infection prevention. The lockdown strategy and the complete closure of workplaces and termination of domestic and international flights were also argued. One study discussed that the decreasing trend of epidemic in Hubei, China, was possibly not driven by the internal travel ban or lockdown [34].
Disease Complications
ARDS is one of the major complications of SARS-CoV-2 infection. A study involving 138 patients in Wuhan, China showed that 19.6% of the patients developed ARDS. Other common complications identified in this study included shock (8.7%), arrhythmia (16.7%), and acute cardiac injury (7.2%). Patients who were admitted and received care in the ICU were more likely to develop these complications than non- ICU patients. Another study including 191 patients in Wuhan, China showed that the most common complication was sepsis (59%) followed by respiratory failure (54%), ARDS (31%), heart failure (23%), and septic shock (20%). Other less frequent complications included coagulopathy (19%), defined as 5-s extension of activated partial thromboplastic time or 3-s extension of prothrombin time, and acute cardiac injury (17%), defined as elevated high sensitivity cardiac troponin I to above the 99th percentile of the upper reference limit or new EKG and/or echocardiogram findings. Non-survivors suffered more of these complications compared to survivors. Interestingly, cardiac events such as new or worsening congestive heart failure, myocardial infarctions, arrhythmias, and cardiac arrest occurred more frequently in patients with associated pneumonia [35].
In severe COVID-19 disease, hypercoagulability can be stimulated by endothelial cell dysfunction, increased blood viscosity from hypoxia, or hypoxia-induced transcription factor-dependent signaling pathway. Acute venous thromboembolism (VTE) has been reported in patients with SARS-CoV-2 infection. A Dutch study involving 184 ICU with proven COVID-19 found a 31% incidence of thrombotic complications, of which 27% comprised of radio graphically, confirmed VTE. Pulmonary embolism (PE) was the most frequent of these thrombotic complications. Another study in Wuhan, China showed that 66 out of the 143 hospitalized patients with COVID-19 included in the study developed a lower extremity deep vein thrombosis (DVT). Their analysis suggested multifactorial causes of DVT in these patients including older age, more severe illness, more chronic illness, stasis, and high thrombotic and inflammatory abnormalities described the case of a 75-year-old COVID-19 positive hospitalized female radio graphically diagnosed with a pulmonary embolism that had no other predisposing factors other than the acute infection with COVID-19 [36].
Risk Factors Associated with Severe Disease
Many studies have shown that severe illness and death occur in patients with certain risk factors including older age and underlying medical comorbidities. A study done by Wu et al. showed that among 44,672 cases of COVID-19 in Wuhan, China, the majority of patients were 30 to 79 years of age (87%) followed by those aged 80 years and older (3%) while only 1% were aged 9 years and younger. Older age was one of the identified risk factors associated with poor prognosis and study showed that those with severe disease were older by a mean of 7 years compared to those with mild disease [37].
It remains unclear whether gender is an independent risk factor for more severe disease. A retrospective case series done in New York showed that among the 393 patients with confirmed COVID-19, 60.6% were males. Also, males were more likely to receive mechanical ventilation. However, this correlation does not imply causation since this study did not adjust for other medical comorbidities [38].
A study by Guan et al. showed that patients with severe disease were more likely to have an underlying coexisting illness compared to those with non-severe disease (38.7 vs. 21%). Another study done in Wuhan, China showed that among 191 patients with COVID-19, hypertension (30%) was the most commonly reported comorbidity followed by diabetes (19%), coronary heart disease (8%), and chronic obstructive lung disease (3%). According to data from the CDC in the US, among 7,162 patients with reported medical problems, diabetes mellitus (10.9%), chronic lung disease (9.2%), and cardiovascular disease (9.0%) were the most commonly reported comorbidities. Immuno compromising conditions (3.7%) and chronic kidney disease (3%) were also reported. In a case series study in New York including 5,700 patients with COVID-19 infection, the most common comorbidities in hospitalized patients were hypertension (56.6%), obesity (41.7%), and diabetes (33.8%). Obesity was found to be risk factor for intubation in a retrospective cohort study of 124 patients with SARS-CoV-2 infection. Of the patients who were intubated, 47.6% had a body mass index (BMI) > 30 kg/ m2 and 28.2% had a BMI > 35 kg/m2 [39].
Case Fatality Rates
According to the Italian National Institute of Health, the CFR in Italy was 7.2% among 22,512 cases up to March 17, 2020. According to data collected by the South Korea CDC, the CFR was 1.79% among 10,237 cases up to April 5, 2020 (KCDC, 2020). According to the CDC in the United States, the CFR was 2.5% among 304,826 cases as of April 5, 2020 (Centers for Disease Control and Prevention, accessed on 22 May2021). The numbers of cases and deaths are evolving on a daily basis; however, it remains unclear why there is such a big difference in CFR across different countries. As noted, the overall CFR in Italy is significantly higher than that reported in China (2.3 vs. 7.2%). The demographic characteristics of the Italian population in 2019 showed that ~23% of its population was above the age of 65. This might somehow explain Italy’s higher CFR compared to other countries affected by the virus with smaller proportions of their populations in this age group. However, when data was stratified according to age groups, the CFR in Italy and China were similar among those aged 0–69 years but the CFR remained significantly higher in Italy compared to China in patients aged 70 years and. Understanding this significant difference in CFR across countries remains challenging and further studies are required to comprehend it fully [40].
Clinical Management and Treatment
Unfortunately, up until this point, there has yet to be a vaccine or proven effective therapy against SARS-CoV-2 infection. While many trials, including much needed randomized controlled trials (RCTs), are currently underway, the mainstay of therapy remains supportive care. This ranges from symptomatic treatment to ventilator support for patients with ARDS depending on illness severity. This also includes recognizing and treating superimposed bacterial infections and/or sepsis early on. Many of the current clinical trials are investigating drugs that were previously used to treat SARSCoV and MERS-CoV. These will be discussed further below.
Chloroquine /Hydroxychloroquine
Chloroquine and hydroxychloroquine are widely used antimalarial drugs. Hydroxychloroquine is a chloroquine analog with less drug to drug interaction and a better safety profile. Both chloroquine and hydroxychloroquine are shown to inhibit the growth of SARS-CoV-2 in vitro and decrease viral replication in a concentration-dependent manner. Hydroxychloroquine was found to be more potent. It has been hypothesized that both chloroquine and hydroxychloroquine may inhibit SARSCoV- 2 replication. They may do this by changing the pH at the surface of the cell membrane thereby inhibiting fusion in addition to inhibiting nucleic acid replication, glycosylation, and viral assembly and release [41].
Multicenter clinical trials in China showed that chloroquine was effective and had an acceptable safety profile in patients with SARS-CoV-2 pneumonia [42]. Hydroxychloroquine is currently under investigation in various RCTs in the Unites States for treatment in patients with SARS-CoV-2 infection and also for pre-exposure and post-exposure prophylaxis. In one retrospective cohort study involving 1,438 patients hospitalized in metropolitan New York, treatment with hydroxychloroquine, azithromycin, or both was not associated with significantly lower in-hospital mortality when compared to neither treatment. However, the interpretation of these findings may be limited by the observational design [43-45].
Azithromycin
Azithromycin is a macrolide antibiotic that has been widely used in patients with chronic pulmonary inflammatory disorders and/or community acquired pneumonia for its antiinflammatory effect. However, there is limited data suggesting the beneficial effect of azithromycin in combination with chloroquine/hydroxychloroquine in the treatment of ARDS in patients with SARS-CoV-2 infection [46].
Remdesivir
Remdesivir is a novel nucleotide analog that incorporates into nascent viral RNA chains and causes premature termination inhibiting viral replication. Remdesivir has been shown to be an effective antiviral agent against beta-coronaviruses such as SARS-CoV and SARS-MERS in mice, non-human primates and in vitro, and is currently in clinical trials for the treatment of Ebola virus [47-49].
Lopinavir-Ritonavir
Lopinavir-ritonavir is a protease inhibitor combination that has been used against human immunodeficiency virus (HIV) infection. This drug was proven to have in. A randomized, controlled, open-label trial that included 199 patients assessed the use of lopinavir–ritonavir treatment in patients with SARS-CoV-2 and showed no benefit with administration of the drug compared to standard care alone, which comprised of antibiotics, vasopressors, renal replacement therapy, extracorporeal membrane oxygenation (ECMO) and/ or supplemental oxygen/invasive ventilation if needed. Gastrointestinal adverse events were higher in the lopinavir– ritonavir group compared to those receiving standard-care alone; however, adverse events were higher in the standardcare group overall [50].
Favipiravir
Favipiravir is an RNA polymerase inhibitor that is used for the treatment of influenza in China. Favipiravir is able to block the replication of RNA viruses by blocking the RNA-dependent RNA polymerase (RdRp) enzyme. Therefore, Favipiravir may have antiviral activity against SARS-CoV-2, which is also an RNA virus. Clinic trials involving the use of this drug in treating SARS-CoV-2 infection are currently ongoing [51].
IL-6 Pathway Inhibitors
As previously mentioned, cytokine storm syndrome and increased levels of IL-6 have been described in patients with severe SARS-CoV-2 infection. IL-6 levels were found to be 2.9-fold higher in patients with severe complicated SARS-CoV-2 infection, including those with ARDS, when compared to mild, non-complicated disease. Until now, there are no RCTs showing that IL-6 inhibitors benefit patients with SARS-CoV-2 infection. However, preliminary investigation demonstrated that IL-6 inhibitors are safe and efficacious in these patients. A single non-randomized, single-arm study showed that patients with severe SARS-CoV-2 infection who received tocilizumab, an IL-6 inhibitor, showed significant clinical improvement including decreased oxygen requirement and resolution of radiographic abnormalities [52]. Treatment guidelines from China’s National Health Commission included tocilizumab for patients with severe SARS-CoV-2 infection who also have increased IL-6 levels based on a multicenter, randomized controlled trial. Multiple IL-6 inhibitors including tocilizumab, sarilumab, and siltuximab are currently under investigation in clinical trials in China [53].
Ivermectin
Ivermectin is an FDA-approved medication for the treatment of various parasites and has an established safety profile in humans. Ivermectin has been shown to inhibit in vitro replication of various positive single stranded RNA viruses such as dengue and west Nile. This drug has recently demonstrated in vitro activity against SARS-CoV-2 when a single dose was able to control viral replication within 24–48 h. It is hypothesized that this is likely through the inhibition of import in α/β1 heterodimer, which mediates nuclear import of viral proteins, a process that many RNA viruses rely on during infection. The FDA has not yet approved Ivermectin for the prevention or treatment of SARS-CoV-2 infection. RCTs studying the efficacy and safety of this drug in COVID-19 are still lacking [54].
Corticosteroids
The use of glucocorticoids in patients with SARS-CoV-2 infection, especially in those with severe disease, was a point of major controversy. The rationale behind their use is to decrease lung inflammation as seen in ARDS. However, this comes with adverse effects such as inhibiting the immune response and thus increasing the risk of secondary infections as well as delaying viral. A Cochran review published in July 2019 that included 48 RCTs found insufficient evidence to determine if corticosteroids were effective at reducing mortality and duration of mechanical ventilation in patients with ARDS [55]. A recent randomized, controlled, open label study known as the recovery trial included 2,104 COVID-19 patients in the United Kingdom (UK) who were randomly allocated to receive 6 mg of dexamethasone per day for up to 10 days compared to standard of care therapy alone [56].
Convalescent Plasma
Convalescent plasma (CP) therapy is a classic adaptive immunotherapy that has been used for decades in the prevention and treatment of various diseases. CP was used in prior epidemics including SARS-CoV, MERS-CoV, and H1N1 in 2009 and it showed successful results with a safe. Given the similarity between SARS-CoV-2, SARS-CoV, and MERS-CoV, CP may have potential efficacy in this current pandemic. However, no RCTs involving CP in SARS-CoV-2 infection have been completed as of yet, and hence the risks and benefits remain unclear [57].
Heparin
As more studies emerge linking coagulopathies to COVID-19 including systemic thrombosis and DIC, this raises the question whether heparin should be used in hospitalized patients to prevent these complications. In a retrospective study in China that included 449 patients, patients who received a prophylactic dose of heparin when they had sepsisinduced coagulopathy (SIC) score ≥ 6 and a d-dimer level >6-fold of upper limit of normal had decreased mortality. Based on the limited available data, the International Society of Thrombosis and Hemostasis (ISTH) recommend the measurement of d-dimer, PT, and platelet count for all patients with COVID-19 infection to help with risk stratification. The society also recommends the administration of low molecular weight heparin at prophylactic dose to all hospitalized patients with no contraindications (RCTs examining the use of heparin in COVID-19 patients are required to make appropriate recommendations [58].
Vitamin C
Vitamin C, also known as ascorbic acid, has antioxidant properties and plays a significant role in reducing inflammatory response. Studies have shown that ascorbic acid downregulates the production of pro-inflammatory cytokines. These concepts have generated interest in the use of ascorbic acid in the management of inflammatory conditions. In a recent randomized clinical trial involving 167 patients in the intensive care unit, intravenous infusion of high-dose ascorbic acid compared to placebo did not significantly reduce organ dysfunction scores or improve levels of biomarkers indicating inflammation among patients with sepsis and ARDS, two disease processes heavily associated with inflammation. A randomized controlled trial is currently underway and in phase 2 to study the clinical efficacy and safety of vitamin C infusion for treatment of COVID-19 pneumonia (Vitamin C Infusion for the Treatment of Severe 2019-nCoV Infected Pneumonia [59].
Zinc
It has been shown that increased zinc concentration inside the cell can effectively impair replication of a number of RNA viruses such as influenza and polioviruses. A study showed that zinc in combination with zinc-ionophores like pyrithione inhibited the replication of SARS-CoV in cell cultures. Therefore, zinc supplementation may be of potential benefit for prophylaxis and treatment of COVID-19 and it is currently under investigation in multiple clinical trials in combination with other agents including hydroxychloroquine, vitamin C, and vitamin D (A Study of Hydroxychloroquine, Vitamin C, Vitamin D, and Zinc for the Prevention of COVID-19 Infection (HELPCOVID-19) [60].
Montelukast
Montelukast has been shown to suppress oxidative stress and have anti-inflammatory effects. Use of high dose montelukast has been effective in the treatment of acute asthma. Because much of the morbidity and mortality from COVID-19 infection is due to excessive inflammatory processes, it is thought that montelukast may play a role in limiting the progression of disease [61].
Mechanical Ventilation and ECMO
For most patients with mild-to-moderate symptoms, bed rest with supportive treatments including sufficient calorie, water intake and maintaining water electrolyte balance, and homeostasis have shown to provide relief. However, for patients with hypoxia, a noninvasive positive airway pressure ventilator could be used. In case of disease severity, the patient is supported with invasive mechanical ventilation with endotracheal intubation. According to WHO-China joint report, about 25% of severe and critical cases require mechanical ventilation while the remaining 75% can be safely supplemented with oxygen only.
Home Care
Home management may be appropriate for patients with mild infection who can be adequately isolated in the outpatient setting. Management of such patients should focus on prevention of transmission to others, and monitoring for clinical deterioration, which should prompt hospitalization. Interim recommendations on home management of patients with COVID?19 can be found on the WHO and CDC websites (Centers of Disease Control and Prevention. Coronavirus disease 2019 (COVID?19): older adults, 2020.
Mesenchymal stem cell therapy
The mesenchymal stem cell (MSC) therapy is also thought to be utilized as they can exert anti-inflammatory and anti-apoptotic effects along with repairing pulmonary epithelial cell damage as well as promoting alveolar fluid damage. Researchers at Lund University, Sweden, developed a lung specific MSC that can reduce lung tissue damage. A pilot study conducted in China showed definitive improvement in seven patients who received MSC treatment. Another study demonstrated that the transplantation of ACE2-mesenchymal stem cells decreased the number of hyper activated CD4+ T and CD8+ T cells and increased the immunosuppressive cytokine IL-10.MSCs were shown to be resistant to SARS-CoV-2 infection and had higher expression of anti-inflammatory factors [62].
Vaccine
WHO lists additional COVID-19 vaccine for emergency use and issues interim policy recommendations
WHO listed the Sinopharm COVID-19 vaccine for emergency use, giving the green light for this vaccine to be rolled out globally? The Sinopharm vaccine is produced by Beijing Bio- Institute of Biological Products Co Ltd, subsidiary of China National Biotec Group (CNBG). WHO also listed the Pfizer/ BioNTech vaccine for emergency use on 31 December 2020; two AstraZeneca/Oxford COVID-19 vaccines on 15 February 2021, produced by AstraZeneca-SKBio (Republic of Korea) and the Serum Institute of India; and COVID-19 vaccine Ad26.COV2.S developed by Janssen (Johnson & Johnson) on 12 March 2021.
• WHO recommendation BioNtech Tozinameran – COVID-19 mRNA vaccine (nucleoside modified) – comirnaty®
• WHO recommendation AstraZeneca/SKBio – COVID-19 vaccine (ChAdOx1-S).
• WHO recommendation Serum Institute of India Pvt Ltd – COVID-19 vaccine (ChAdOx1-S) – COVISHIELD.
• WHO recommendation Janssen–Cilag International NV (Belgium) COVID-19 vaccine (Ad26.COV2-S).
• WHO recommendation AstraZeneca/EU approved sites COVID-19 vaccine (ChAdOx1-S).
• WHO recommendation Moderna COVID-19 mRNA vaccine (nucleoside modified)
• WHO recommendation COVID-19 vaccine BIBP/ Sinopharm.
Ongoing Vaccine Trials
The development of vaccine is in progress, but it is estimated that a working safe vaccine will require at least 12–18 months being available for wider use. There are mainly four types of vaccine strategies used for COVID-19 to provoke immune response: first, using either a weakened or inactivated virus; second, using either replicating or non-replicating viral vectors, to produce viral proteins inside the body; third, using nucleic-acid-based vaccines, either RNA or DNA, to make copies of viral spike protein using host machinery; fourth, using protein-based vaccines to inject viral protein fragment or virus-like particle inside the body. Around 90 vaccines are undergoing active development and some of them have already started safety trials. The majority of the vaccines are designed to target the viral spike protein as it is the major inducer of neutralizing antibodies. The Ad5-nCoV is in phase- II in China and ChAdOx1-nCoV is preparing to enter phase-II trial in the UK. The primary result from the recombinant Ad5- nCoV vaccine expressing the spike glycoprotein showed to stimulate humoral response and the vaccine was found to be safe and tolerable.
Vaccine Delivery Systems
Liquid-based intramuscular needle-and-syringe injections have a few caveats. They are expensive to store and transport; many of them are sensitive to temperature. Some advanced approaches are in active development for COVID-19 such as the Langerhans cell targeted delivery system (LC-TDS) (Corona: Vaccination without a needle? Max-Planck- Gesellschaft, Munich, Germany, 2020, The LC-TDS employs micro needle patches containing specific ligands embedded in liposomes that are uptaken by the Langerhans cells in the skin, thereby stimulating immune response. The microneedle patches quickly dissolve and allow painless, injury-free administration of vaccines. Other options that can be explored in vaccine delivery include solid dose vaccine delivery system based on mucosal route to present antigens to mucosa associated lymphoid tissues (MALT). The vaccines currently available via oral route include rotavirus, typhoid, cholera, and poliovirus. Moreover, a live attenuated typhoid vaccine Ty21a is available as orally delivered capsule that can be readily ingested.
A Second Wave
As seen in multiple previous pandemics including the influenza pandemic of 1918, the first wave is often followed several months later by a second wave of infections that could potentially be even worse than the first. A second wave can be caused by a region being re-exposed to infection by an influx of infected people from another. The degree of the resulting new outbreak will depend on the level of immunity in the first region from the initial wave. This will be influenced by multiple factors including the potential for endogenous loss of immunity in the first population and the introduction of people who are not immune, for example, individuals moving from one state to another in the US to date, mitigation strategies have been effective at controlling the pandemic in several regions, a study by Aleta et al.
Tackling the Second Wave
As countries around the world have started easing down on the previously imposed restrictions such as opening up businesses and shops and allowance for travel, there is an increased risk for a second wave of infection, which would be difficult to contain, especially during the winter season. Premature termination of strict measures could potentially lead to a second wave of infection. One modeling study demonstrated that relaxing the lockdown too early would cause the R0 to exceed 1 and spread across China. Epidemiologic disease modeling could play a vital role by predicting the plausible infection rate status. Modern technology such as machine learning could be used to process a large amount of data and generate better.
Lessons Learned for Future Pandemics
As this pandemic continues to develop and continues to take the lives of so many, there are innumerable lessons to be learned for future pandemics. To begin with, it is crucial to establish clear whistleblowing policies for potential global health emergencies. This will allow for transparency and help encourage clinicians to bring important information to light as soon as they are detected. Once high-risk areas have been identified, precautions including travel restrictions and quarantines should be implemented as soon as a possible health threat is identified. Also, framework should be developed to escalate a threat status earlier for fast-spreading diseases.
The Third Wave of the COVID-19
The second wave of COVID 19 has caused lakhs of lives in India and millions across the globe. In case you are infected during the third wave of coronavirus, you may face shortness of breath, cough, fever and breathing difficulties. According to the opinion of the health department, such symptoms may take approximately 2-14 days to appear in a person’s body. It is always recommended to immediately get in touch with medical experts or a genuine and reliable pathology lab once you notice these symptoms getting visible in you or any of your loved ones.
Precautions to be taken during the third wave
• Wearing a mask while stepping outside is mandatory for each and every one. However, it is also recommended to wear more than one mask for better protection.
• Take enough time to wash your hands in a proper manner soon after you come back home from the outside. Keep a hand wash prepared for everybody who is entering your house from the outside.
• Use hand sanitizers every time you wear any random object or surface. Always keep this in mind that your silly carelessness can be the reason for a massive sickness of yourself or any of your family members, which may even turn fatal.
• Try not to take public transport while going somewhere. It is always recommended to stay away from crowded zones to avoid getting transmitted.
Forth Wave of COVID-19
After some reprieve, the world is once again witnessing the sudden surge in Covid-19 cases across many countries. Omicron subvariant BA.2, also known as Stealth Omicron, is said to be behind the 4th wave of the Covid-19 pandemic. Deltacron variant however is said to be not behind the spurt in COVID cases. Doubts have arisen about the fourth wave in India as the cases of Omicron sub-variants increase. Health Minister of Karnataka and IIT Kanpur has already warned that the 4th wave in India could come in the month of August. At the same time, experts have described two symptoms of Omicron sub-variant as the main
What is BA.2?
A sub lineage of the Omicron family has three sub variants - BA.1, BA.2 and BA.3. BA.1 was the original Omicron variant that took off and spread around the world, being highly transmissible. But now, BA.2 also known as Stealth Omicron has gained a foothold in many countries, leading to a rises in case numbers. One of the most interesting things about the three sub variants is that each is as different to the other as Alpha, Beta, Gamma and Delta are from each other. The three Omicron sub lineages, which were detected at the same time in South Africa, share 39 mutations. But each has some of their own mutations too. In the case of BA.1 there are 20, BA.2 has another 27 and BA.3 has 13 more. BA.2 spreads faster than BA.1, about 30% more according to estimates, which means it is easier to catch. Scientists think its extra mutations help to make it more transmissible. "Omicron BA.2 is about 1.4 times more infectious than BA.1," wrote former World Health Organization scientist Prof Adrian Esterman on Twitter.
Stealth Omicron symptoms
1. Many researchers suggest Omicron sub-variant BA.2 has two special characteristics, which includes dizziness and extreme tiredness.
2. Both symptoms can appear within two to three days of being infected with the virus and can last longer in infected patients than before.
3. BA.2 sub-variant or Stealth Omicron affects more on the stomach and intestines of the people infected, leading to digestive problems.
4. Infected patients are complaining of six gut-related sicknesses - nausea, diarrhoea, vomiting, abdominal pain, heartburn and bloating.
5. Loss of appetite, back pain, abdominal bloating, and swelling of the intestine, body cramps and depression are other symptoms faced.
6. Besides, other symptoms include fever, cough, sore throat, and blood clot formation in the head, muscle and joint pain, high blood pressure.
7. Persons infected with Omicron BA.2 sub-variant do not face symptoms like loss of smell or taste and shortness of breath.
Omicron symptoms
Fever or chills, Cough, Shortness of breath or difficulty breathing, Fatigue, Muscle or body aches, Headache, New loss of taste or smell, Sore throat or hoarse voice,Congestion or runny nose, Nausea or vomiting, Diarrhoea, High temperature, Continuous cough, Back pain, A loss of appetite, Heartburn, Bloating, Sleep paralysis , Skin rash
Conclusion
The pandemic of COVID-19 has challenged our existing knowledge, laws, and regulations and forced us to take measures as far as complete lockdown in various parts of the world. The high death toll of COVID-19 has stressed the need for prompt research and dissemination of updated information. This review summarized the scopes and developments of COVID-19 diagnosis tools and therapeutic options and discussed the prevention and control measures considering an apparently upcoming second wave of infection. While the world is in search for a cure, it is recommended that countries make use of existing scientific tools to develop models to predict community-based outcomes prior to making decisions. Healthcare workers must be supported with supplies and remain updated with the up-to-date knowledge, and citizens must play their role to maintain basic guidelines. At the governmental level, facilitating more testing and contact tracing, providing timely publication of epidemic information, enabling early diagnosis, and delivering supportive treatments for the patients are of utmost importance.
References
- De Wit E, Van Doremalen N, Falzarano D, et al. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-34.
- Sanche S, Lin YT, Xu C, et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerging Inf Dis. 2020;26(7):1470.
- Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. New Eng J Med. 2020;382(10):970-1.
- Mahévas M, Tran VT, Roumier M, et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalised for COVID-19 infection and requiring oxygen: results of a study using routinely collected data to emulate a target trial. medrxiv. 2020.
- Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193-292.
- https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions.
- Soni A, Kumar A, Kumar A, et al. New Era of Formulation as Silver Nanoparticles in Pharma. J Drug Delivery and Therapeutics. 2021;11(2-S):126-31.
- https://www.sciencedaily.com/releases/2020/11/201102173232.htm
- Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. Jama. 2020;323(14):1406-7.
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Resp Med. 2020;8(5):475-81.
- Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol: J Pathol Soc Great Brit Ireland. 2004;203(2):631-7.
- Mossel EC, Wang J, Jeffers S, et al. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virol. 2008;372(1):127-35.
- Kumar A, Kumar A, Soni A, et al. Development and In-Vitro Evaluation of Itraconazole Loaded Nanoemulsion. J Drug Delivery Therapeutics. 2022;12(3):31-42.
- COVID MA. infection: Emergence, transmission, and characteristics of human coronaviruses/Muhammad Adnan Shereen, Suliman Khan, Abeer Kazmi, Nadia Bashir, Rabeea Siddique. J Adv Res. 2020;24(3):91-8.
- Wang X, Cao R, Zhang H, et al. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell discovery. 2020;6(1):1-5.
- Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Inf. 2020;81(1):e21-3.
- Chen C, Zhang Y, Huang J, et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. MedRxiv. 2020.
- Lian N, Xie H, Lin S, et al. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clinical Microbiol Inf. 2020;26(7):917-21.
- Huang C, Wang Y, Li X, et l. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497-506.
- Ravi N, Cortade DL, Ng E, et al. Diagnostics for SARS-CoV-2 detection: A comprehensive review of the FDA-EUA COVID-19 testing landscape. Biosensors and bioelectronics. 2020;165:112454.
- Azhar M, Phutela R, Ansari AH, et al. Rapid, field-deployable nucleobase detection and identification using FnCas9. BioRxiv. 2020.
- Joung J, Ladha A, Saito M, et al. Point-of-care testing for COVID-19 using Sherlock diagnostics. MedRxiv. 2020.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020;323(18):1843-4.
- Yan L, Zhang HT, Xiao Y, et al. Prediction of criticality in patients with severe Covid-19 infection using three clinical features: a machine learning-based prognostic model with clinical data in Wuhan. MedRxiv. 2020;27:2020.
- Henrina J, Putra IC, Lawrensia S, et al. Coronavirus disease of 2019: a mimicker of dengue infection?. SN Comprehensive Clinical Med. 2020;2(8):1109-19.
- Imran A, Posokhova I, Qureshi HN, et al. AI4COVID-19: AI enabled preliminary diagnosis for COVID-19 from cough samples via an app. Informatics in Med Unlocked. 2020;20:100378.
- https://www.bsigroup.com/en-GB/topics/novel-coronavirus-covid-19/medical-devices-ppe/.
- Cook TM. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic–a narrative review. Anaesthesia. 2020;75(7):920-7.
- Zhang Y, Jiang B, Yuan J et al. The impact of social distancing and epicenter lockdown on the COVID-19 epidemic in mainland China: A data-driven SEIQR model study. MedRxiv. 2020.
- Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12(3):254.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet. 2020;395(10229):1054-62.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New Eng J Med. 2020;382(18):1708-20.
- Rate CF. Characteristics of patients dying in relation to COVID-19 in Italy Onder G, Rezza G, Brusaferro S. JAMA Published online March. 2020;23.
- Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. Jama. 2017;318(4):360-70.
- Musinguzi G, Asamoah BO. The science of social distancing and total lock down: does it work? Whom does it benefit?. Electronic J General Med. 2020;17(6).
- Koo JR, Cook AR, Park M, et al. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study. The Lancet Infec Dis. 2020;20(6):678-88.
- Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thrombosis Haemostasis. 2020;18(5):1094-9.
- Ku CC, Ng TC, Lin HH. Epidemiological benchmarks of the COVID-19 outbreak control in China after Wuhan’s lockdown: a modelling study with an empirical approach. Available at SSRN 3544127. 2020.
- Danzi GB, Loffi M, Galeazzi G, et al. Acute pulmonary embolism and COVID-19 pneumonia: a random association?. Europ Heart J. 2020;41(19):1858-.
- Flegal KM, Graubard BI, Williamson DF, et al. Cause-specific excess deaths associated with underweight, overweight, and obesity. Jama. 2007;298(17):2028-37.
- Geppert CM. Trust in a Vial. Federal Practitioner. 2021;38(1):4.
- Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York city. New Eng J Med. 2020;382(24):2372-4.
- Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. The Lancet Infect Dis. 2020;20(6):669-77.
- Dighe A, Cattarino L, Cuomo-Dannenburg G, et al. Response to COVID-19 in South Korea and implications for lifting stringent interventions. BMC Med. 2020;18(1):1-2.
- https://www. cdc. gov/coronavirus/2019-ncov/index. html Accessed. 2020 Jul;25.
- Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clinical Infect Dis. 2020;71(15):732-9.
- Paumgartten FJ. The ethical limits of physicians’ autonomy and the Brazilian Federal Council of Medicine. Cadernos de Saúde Pública. 2022;38.
- Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid–sparing effect of benralizumab in severe asthma. New Eng J Med. 2017;376(25):2448-58.
- Amsden GW. Anti-inflammatory effects of macrolides—an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions?. J Antimicrobial Chemotherapy. 2005;55(1):10-21.
- da Silva BN, Faria AR, Souza SD, et al. Expression of VanA-type vancomycin resistance in a clinical isolate of Enterococcus faecium showing insertion of IS19 in the vanS gene. Int J Antimicrobial Agents. 2020;55(4):105897.
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-71.
- Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. New Eng J Med. 2020;382(24):2327-36.
- Groneberg DA, Poutanen SM, Low DE, et al. Treatment and vaccines for severe acute respiratory syndrome. The Lancet Infect Dis. 2005;5(3):147-55.
- Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discoveries Therapeutics. 2020;14(1):58-60.
- Coomes EA, Haghbayan H. Interleukin?6 in COVID?19: a systematic review and meta?analysis. Rev Med virol. 2020;30(6):1-9.
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. The lancet. 2020;395(10229):1033-4.
- Tay MY, Fraser JE, Chan WK, et al. Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Res. 2013;99(3):301-6.
- Yang SN, Atkinson SC, Wang C, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177:104760.
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The lancet. 2020;395(10223):473-5.
- Hirko KA, Kerver JM, Ford S, et al. Telehealth in response to the COVID-19 pandemic: Implications for rural health disparities. J Am Med Informat Assoc. 2020;27(11):1816-8.
- Mouritsen J, Larsen HT. The 2nd wave of knowledge management: The management control of knowledge resources through intellectual capital information. Management Accounting Res. 2005;16(3):371-94.
- Bhattacharya PK. RE: Delta Plus variants Corona virus in India: What may be the Impact in expected 3rd wave.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref