Commentary - Asian Journal of Biomedical and Pharmaceutical Sciences (2022) Volume 12, Issue 90
Comparative review of the technological and biochemical basis behind four different COVID-19 vaccines produced in the United States and Europe.
Evance Okoyo*
Department of Biological Sciences, University of Eldoret, Eldoret, Kenya
- *Corresponding Author:
- Evance Okoyo
- Department of Biological Sciences
University of Eldoret
Eldoret
Kenya
E-mail: evanceokoyo@gmail.com
Received: 28-April-2022, Manuscript No. AABPS-22-62132; Editor assigned: 03-May-2022, PreQC No. AABPS-22-62132(PQ); Reviewed: 18-May-2022, QC No. AABPS-22-62132; Revised: 26-May-2022, Manuscript No. AABPS-22-62132(R); Published: 03-June-2022, DOI:10.35841/2249-622X.90.129
Citation: Okoyo E. Comparative review of the technological and biochemical basis behind four different COVID-19 vaccines produced in the United States and Europe. Asian J Biomed Pharmaceut Sci. 2022;12(90):129
Abstract
The advent of COVID-19 arising from SARS-CoV-2 has interrupted the world to unbelievable levels. To establish a common immunity and subsequently safeguard humanity, several laboratories across the world have developed a couple of vaccine formulations with the sole intention of fighting COVID-19, a ravaging respiratory tract infection that has interrupted the usual norms worldwide. The vaccines considered, Johnson & Johnson, AstraZeneca, Modern, and Pfizer, qualitatively depict diverse ways of biochemically shielding the body, consequently different efficacy. This paper provides a comprehensive comparative review of existing information on the biochemical features and technology of the four COVID-19 vaccines. Explicitly, little scientific documentation to date has carried out a detailed review of the available COVID-19 vaccines simultaneously, hence offering a comparative analysis. The article is a summary of valuable information regarding the biochemical composition and technology behind the above-discussed vaccines, hence shedding more light on the safety and efficacy parameters on each. However, it is not meant to be a complete review of the subject matter.
Keywords
Spike protein, COVID-19, Vaccine, mRNA, Vector virus.
Introduction
In late 2019, the international public health agency, World Health Organization (WHO) got a report about new cases of virus-related pneumonia of unidentified origin in China. In 2020, the pathogen causing this abnormal disease was acknowledged as severe acute respiratory coronavirus 2 (SARS-CoV-2) – a novel family of coronaviruses. Its genome was sequenced and the resultant data published. Since then, the novel virus spread to every region in the world, prompting WHO to mark it as a public health challenge of worldwide concern. The outbreak was consequently declared a pandemic in March 2020 by WHO. The pandemic is continuing despite exceptional efforts to curb the outbreak.
While many countries globally have revolutionized their health systems in recent years, few clinical challenges could turn so critical and pertinent to the international attention that their occurrence result in radical transformation in all echelons of human life. The infective virus, SARS-CoV-2 that results in COVID-19 possesses an immense threat facing global health systems. Moreover, the mutational situation in which the pathogen acquires distinctive and persistent evolutionary features further compounds its management. The emergence of novel coronavirus types made it hard for prevailing healthcare systems to react quickly to the incipient threat following little experience in handling this pathogen. In turn, the development of SARS-CoV-2 interrupted the international agenda, showing the feeble nature of health systems against the advent of new diseases.
COVID-19 has not only affected the health sector but also hurt all spheres of public life where deep casual links are freely apparent. Incapable to contend with dramatically high numbers of crisis patients, health facilities were virtually stunned and unproductive in assisting to manage the insistent pace of the ravaging pandemic. Most governments were compelled to enforce severe containment measures such as lockdowns, banning social gatherings, and public facilities among other things [1]. The situation also resulted in ruining small and medium enterprises, making several businesses to close and even disrupt existing supply chains. In addition, to drastically contain the swift spread of the virus, numerous nations hurriedly closed borders and suspended visa issuance on the context of protecting national security. Evidently, due to the novel threat, every range of life has come under intense pressure, meaning public life, anchored in the concept of globalism, has become sore.
Information about the Coronavirus
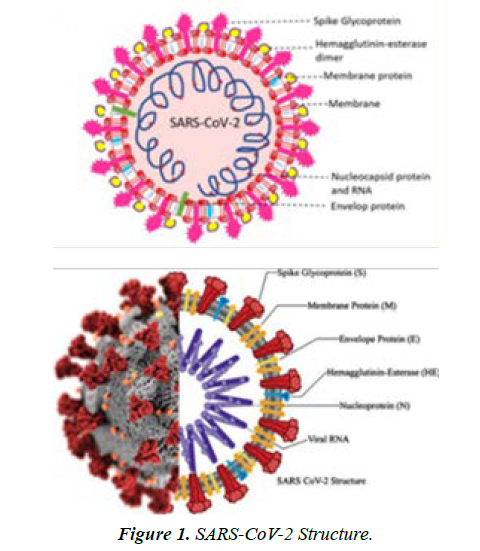
Coronaviruses are a well-studied group of viral particles containing RNA molecules as genetic material. The name of this family was given to viruses solely because of the similarity of their morphological structure to the solar corona. The lipoprotein envelope of the virus has several spike-like peplomers designed for efficient binding to cell receptors, as shown in Figure 1. SARS-CoV-2 is entirely consistent with this description and appears to be a naturally occurring virus, although this position still has no clear academic assessment [2]. A central argument in the assumption of a natural origin of the virus causing COVID-19 is the high similarity rate to SARS-CoV, which caused the SARS epidemic in 2002 [3]. Alternatively, genomic studies of the structure of the virus reveal some inaccuracies and assumptions that allow opponents of the natural origin of the pathogen to discuss its laboratory production. For example, the presence of a viral laboratory at the epicenter, Wuhan, China, and the phylogenetic differences from the progenitors, and the multiplicity of mutated forms provide the basis for skepticism. At the same time, the current virus has a steady tendency to evolve through mutagenic processes of genetic material. This creates a wide variety of similar pathogens with various functionalities and makes it much more challenging to fight them effectively.
According to WHO, as of 12th April 2022, there are about 497 million reported occurrences of COVID-19 worldwide with approximately 6.2 million reported deaths resulting from this disease. Moreover, in the same period under review, WHO reports that a total of 6 billion vaccine doses have been administered worldwide. Notably, huge chunk of infection leads to mild or asymptomatic disease with full recovery usually reported. Other underlying health conditions like diabetes, hypertension, chronic respiratory disease, cardiovascular disease, cancer, obesity, and chronic kidney disease are regarded as possible risk aspects for contracting dreadful COVID-19. Chromosomal abnormalities, increasing age, and organ transplant also remain other risk factors (Figure 1).
Morphological characteristics
Typically, SARS-CoV-2 is a positive-sense single-stranded RNA (+ssRNA) virus having a singular liner segment of RNA. According to [4], the virus is enveloped and contains virion, which has smaller diameters ranging between 50 and 200 nanometers. SARS-CoV-2, just like other types of coronaviruses has four structural proteins namely, S (spike), N (nucleocapsid), E (envelope), and M (membrane). The spike protein has a polybasic cleavage location, a feature recognized to enhance transmissibility and pathogenicity in other viruses. The spike protein helps the virus to fuse with and attach to host cell’s membrane.
The spike protein’s S1 subunit catalyzes attachment of the virus to the angiotensin-converting enzyme 2 receptors inside the respiratory system cells. The S2 and S2’ subunits expedite the binding of the novel virus with the host cell membrane. The S protein is regarded an appropriate antigen in the development of vaccine since research demonstrates that antibodies channeled against it neutralizes the virus thereby generating an immune reaction that averts infection in target animals. It is thought that SARS-CoV-2 has zoonotic background with close genetic resemblance to bat coronaviruses. The virus belongs to the beta coronaviruses. In addition to these proteins, the viral particle also has biopolymers that form the nucleocapsid envelope.
Such proteins package the viral RNA and play a fundamental role in the assembly of the viral particle, but the structure of the coronavirus nucleocapsid is not currently described in any detail [5]. From the known information, it should be noted that the internal proteins of the capsid are bound in a chain no thicker than fifteen nanometers, with the capsid as a whole not occupying the entire free volume of the pathogenic particle. Inside the nucleocapsid, as is customary for viruses, there is genomic material.
Etiological evidence and pathogenesis
Human-to-human spread of SARS-CoV-2 was established around January 2020. SARS-CoV-2 is a pathogen of the respiratory system of humans. Its transmission takes places majorly via respiratory droplets from sneezes and coughs and through aerosols. Following an infection, a person can remain infectious for about two weeks and can transmit the virus even when they do not manifest the symptoms. The average incubation duration following an infection to the time of showing symptoms is about five days.
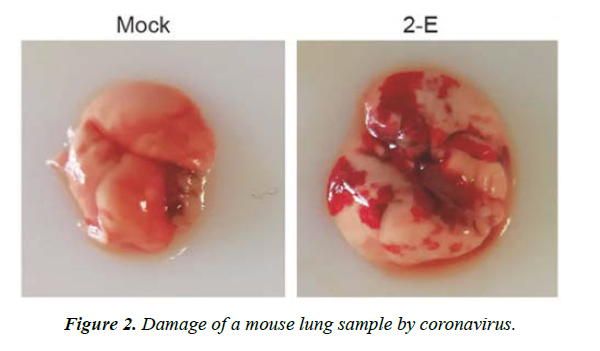
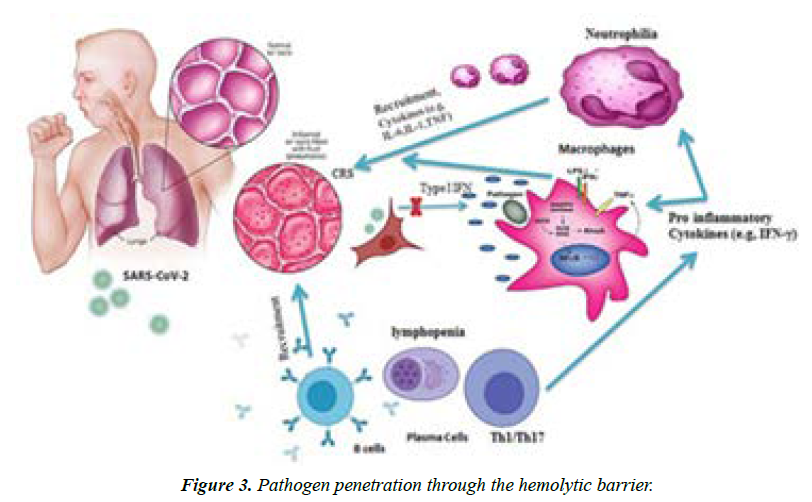
Moreover, most symptomatic persons depict symptoms between second- and eighth-day following exposures while nearly every symptomatic person will encounter at least one symptom before the twelfth day. Common symptoms encompass cough, fever, breathing difficulties, loss of taste and smell, fatigue and the symptoms might change over time. The virus is capable of causing acute respiratory viral infections, including respiratory failure, as shown in (Figure 2) [6]. The critical entry gates for pathogen entry are the epithelium of the upper respiratory tract. SARS-CoV-2 first attaches to target cells that have ACE2 receptors, including in the cells of the nasopharynx (Figure 3).
As a result, a change in a patient's sense of smell at an early stage of the disease may be indicative of nasopharyngeal mucosal edema. However, each of the existing kinds of SARSCoV- 2 shows exclusive admission to the host; so many facets of pathogenesis need further critical study. A study conducted by ECDC reveals histologic outcomes from the lungs to encompass diffuse alveolar harm akin to lung damage inflicted by viruses like those of MERS-CoV and influenza. Another unique feature of the SARS-CoV-2 infection entails vascular injury, with serious endothelial damage, angiogenesis, micro angiopathy, and widespread thrombosis.
COVID-19 clinical presentation and diagnosis
The presentation and brutality of COVID-19 differs among individuals. It can depict mild symptoms, reminiscent of some upper respiratory conditions such as the common cold. Patients with critical or severe COVID-19 characteristically improve between three and six weeks. Some mild cases may take around two weeks to recover. Elevated C-reactive protein amounts and prolonged prothrombin time on hospital admission are linked to harsh cause of the disease. The acceptable standard technique of testing COVID-19 is through the reverse transcription polymerase chain reaction (RT-PCR) due to its effectiveness in spotting the existence of viral RNA fragments. Since this test identifies RNA rather than infectious virus, its capacity to establish the length of contagion in the infected persons becomes inadequate. The RT-PCR test is typically performed on samples of nasal or nasopharyngeal swab. It can also be done on the sputum sample.
Vaccination as a solution to the COVID-19 problem
Every period when human beings are faced with a novel infectious disease, there are concerted efforts from clinical laboratories to establish efficient diagnostic tests, vaccines, and even effective cure. A vaccine to trigger the development of an artificial immunity is a tremendous solution that has been confirmed over time by scientists from multidisciplinary orientations and numerous generations of physicians. Notwithstanding, the most effective vaccine needs to be derived using contemporary instrumental biotechnology techniques and offer a specific reaction to a given virus strain. In order to accomplish these goals, various technologies have been advanced and accepted by global responsible authorities for approving vaccine materials – from mitigated, non-pathogenic pathogens to recombinant vectors having genetically modified copies of the virus antigens. Explicitly, this work carries a comparative review of most popular forms of vaccine that have largely gained public acceptance on the global stage: Moderna, Pfizer, Johnson & Johnson, and Astra Zeneca.
The comparative review of the vaccine phenomenon
Among the existing clinical tools for controlling infectious diseases, vaccines should be given special attention. In contrast to classical drug treatment, vaccination is a preventive therapy to generate an immune response. The general pattern of forming an immune response through vaccines is the same. By injecting a foreign antigen into the patient's body, it produces its antibodies, making it easier to recognize and capture the pathogen if future infections. Each vaccine has a targeted effect against a specific infectious agent since the mechanism of action of the drugs aims to create antibodies to specific antigens. On the other hand, vaccines differ in their methodological approaches to creation: either whole viruses or their fragments can be used as the critical protective agent. This literature review examines various aspects of the vaccination phenomenon. The review focuses on the specific vaccine products with the greatest international acceptance as a means of controlling COVID-19 infection, namely Pfizer, Moderna, Astra Zeneca, and Johnson & Johnson.
Variety of Vaccines
Vaccines were thought to be defined as introducing only a dead, weakened pathogen into the human body to make the body’s immune system establish a deterrent response to the infectious agent. In reality, this information is outdated, as the methodology of modern vaccines has evolved considerably [7]. Current biotechnological advances make it possible to obtain vaccines from at least two different technologies. This concerns both the injection of whole attenuated pathogen and a more innovative scheme to introduce its key fragments. Attenuated vaccines are a historical legacy of the last century, but within the current reality, the use of attenuated pathogens is often considered more expensive, unreliable, and difficult to transport and store biological material.
Moreover, the technology itself for producing such a vaccine loses significantly in clinical safety, as lab technicians must have a constant supply of active viruses to obtain attenuated material. This in itself creates high pressure on the technical equipment requirement of the laboratory. The virus attenuation procedure is standardly implemented through mutation initiation, and therefore the selection of competent vectors becomes more difficult. An additional threat to the method is the inability to use the drug in immune-compromised patients, whether HIV-infected, pregnant, elderly, or children.
The immune system can create an effective protective response to a whole pathogen and its fragments. This assertion is based on a thorough understanding of the causal mechanisms that determine the formation of the immune response. For example, when a pathogen enters the body with an antigen, antibodies are formed by blood B-cells through a series of cascade reactions [8]. There is no need to inject the whole virus, but fragments of its active parts that trigger an immune response are sufficient. For this purpose, a surface protein is used, which is produced in vast quantities using genetic engineering methods. Notably, low-molecular-weight proteins are rarely immunogenic, so manufacturers use antigen-carrying vectors harmless to the human body. As can be seen, this method is considered to be safer for the human body, but the genetically engineered part of it requires significant time resources.
Genetic characteristics of Vaccines
The vaccines actions are anchored in the interaction of the existing genetic material of the injected drug with the internal structures of the immune cells. Depending on which genetic molecule forms the basis of the vaccine, DNA or mRNA, there are two different vaccine technologies. In vector adenovirus vaccines, the drug injection contains third-party viruses that cannot replicate and are equipped with copies of SARS-CoV-2 genes. The list of such vaccines includes Astra Zeneca and Johnson & Johnson: the drugs are injected intramuscularly, from where they enter the immune cells and start the triggering mechanisms of biosynthesis of the S protein of the coronavirus.
On the alternative side are mRNA vaccines, which have an RNA molecule. For example, Moderna and Pfizer vaccines deliver ready-made instructions to the target cells to synthesize a protein antigen, in response to which the human immune system produces its antibodies. Thus, unlike AstraZeneca and Johnson & Johnson, Pfizer and Moderna vaccines do not require the use of defective viruses and thus are suitable for more immuno compromised people. Consequently, mRNA vaccine production can be more extensive and adaptive: this is especially true in mutant strains. Finally, the clearest gap between the two types of vaccines used is how precisely the immune response is formed.
Unlike vector adenovirus vaccines, mRNA vaccines can be administered an unlimited number of times since the patient's immune system creates a response to the adenovirus vector, meaning that repeated administration of such a DNA vaccine would be ineffective. This leads to a necessary practical consequence: if the patient has already had an infection with this adenovirus before, the vector vaccine will not help him.
The essence of the two types of Vaccines
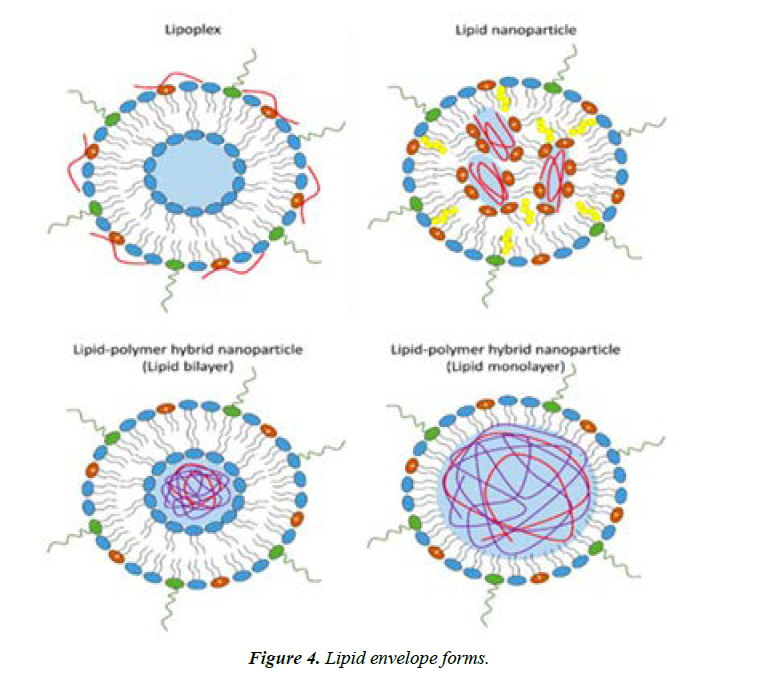
The composition of mRNA vaccines is the same since the preparations contain a lipid shell and genetic material. Pfizer and Moderna present a lipid capsule having a fragment of mRNA for the encoding the S-protein in the SARS-CoV-2 virus [9]. The lipid envelope is not only to protect the internal contents from destruction but also to ease the entry of the component of Pfizer and Moderna into the cell by absorbing the lipid particle into the cell (Figure 4).
The mRNA vaccine will stay in the target cell in the enveloped state until the lipid capsule is destroyed due to changes in the pH of the environment. According to, the conditions for mRNA encapsulation must necessarily be weakly acidic to break down the fat barrier of the vaccine chemically. Once the foreign genetic material leaves the envelope, the phase of active protein biosynthesis begins: mRNA enters the endoplasmic reticulum, where protein translation occurs under the action of rRNA. Subsequently, such proteins are released outside the cell using the functions of the Golgi apparatus, after which the immune system, triggers the process of biochemical protection against the foreign protein.
Concerning Pfizer and Moderna, it should be said that the immunogenic, provocative function is performed not by the added adjuvants but by the lipid membrane itself. In other words, the lipid capsule of the mRNA vaccine is modified to simplify the biochemical composition of the preparation.
The fundamental choice of adopting the S-protein for targeting Moderna and Pfizer vaccines is not accidental. SARS-CoV-2 has been shown to use S-proteins to bind to cell receptors, and this mechanism is implemented via the RBD domain on the peplomer that recognizes the ACE2 receptor. Consequently, blocking these S-proteins by antibodies previously generated from plasma cells results in the inability of SARS-CoV-2 to actively bind to the target cell. Notably, the nature of the antibody-protein S-protein bond is based on a unique epitopeparatope interaction in which a particular antibody specifically and selectively binds to the pathogen antigen [10].
In this context, it is essential to note that the molecular size of the S-protein is 1273 amino acids, while the size of the RBD domain does not exceed 222 amino acids. Considering that only a few amino acids are sufficient to connect the epitope to the paratope, it is relevant to emphasize that each S-protein has a whole host of epitopes for binding to the ACE2 receptor. As a result, the immune system must develop unique antibodies to eliminate the possibility of infection in future attacks.
In addition to next-generation vaccines, technically more sophisticated drugs such as AstraZeneca and Johnson & Johnson are gaining widespread popularity. The point of the biochemical action of such vector vaccines is to introduce into the body a DNA virus from which the E1 and E3 genes have been previously removed, and an S-protein-producing gene added instead. AstraZeneca uses the recombinant ChAdOx1 virus as the adenovirus, while Johnson & Johnson uses rAd26 to stabilize conformation.
As a result, the adenovirus vector vaccine uses only natural mechanisms of immune system activation, so it is especially important that it cannot weaken the immune system in any way. The vector preliminarily restricts the ability to replicate, so the individual should not get sick with adenovirus infection, much less coronavirus. Unlike mRNA vaccines, vector vaccines require less stringent conditions to maintain their biological activity. AstraZeneca has been shown to use the preservative EDTA to preserve the conformational activity of proteins.
There are opinions that EDTA can provoke thrombosis in inoculated patients, but there is insufficient reliable evidence to suggest that this is true. Occurrences of thrombosis have also been characteristic of Johnson & Johnson vaccinated patients. It has been reported that in the case of these vector vaccines, the possibility of coronavirus proteins entering the nucleus of the target cell leading to the death of the latter has not been excluded. As a result, such fragments entered the bloodstream and led to the formation of lethal clots. Notably, Pfizer and Moderna vaccines based on a different mechanism did not lead to thrombosis, which means that the problem may indeed lie in need for the viral gene to be transcribed in the host cell [11].
Intermediate efficacy of Vaccines
Most approved vaccines are still being clinically evaluated on a large sample of people, gathering information about possible side effects and adverse events. For this reason, the effectiveness of the vaccines is estimated to be relative, not finite. Pfizer was initially rated at 95% efficacy in suppressing symptomatic disease when approved by the FDA, with efficacy remaining at 90% when fully immunized. Against new strains of SARS-CoV-2, Pfizer's efficacy was only 88%.
The proven efficacy of Moderna is slightly lower at 94.1%, with efficacy decreasing to 90% for complete immunization. Johnson & Johnson's estimated efficacy is only 72% and 86% for severe disease. Finally, the overall efficacy for the Astra Zeneca vector vaccine is less than 76% and 100% for therapy of complicated cases. In general, without a plausibility analysis, it can be seen that mRNA vaccines appear to be more effective compared to adenoviral, which have, among others, cases of severe side effects [12].
As this literature review has shown, two lines of vaccines for the formation of artificial immunity against COVID-19 are currently relevant. Vector vaccines use weak viral agents that transfer the gene of interest into human cells. mRNA vaccines are more advanced forms and are represented by a lipid shell having genetic material. The proven efficacy of mRNA vaccines is comparably higher, with less overall evidence of possible adverse events for such drugs. Thus, vaccines such as Pfizer and Moderna show better performance compared to Johnson & Johnson's and AstraZeneca.
Moderna is one of the expedited vaccines developed to prevent COVID-19. The basis of the vaccine is the nucleoside-adapted mRNA translating for the whole length virus’s spike protein changed at the 2 proline replacements in the heptad recurrence 1 sphere denoted by S-2P to help in stabilizing the spike protein for the perfusion conformation. Lipid nanoparticles (LNP) encapsulate mRNA. The main role of the spike protein is to facilitates the virus’s entry and attachment to the new host cells by binding with ACE2, placing it as main target for defusing antibodies that avert infection.
After delivery and acceptance by the body cells, translation of the mRNA occurs at the cytosol prompting the generation of spike protein by the machinery of the host cell. Moreover, the presented spike protein produces an adaptive cellular and humoral reply. The neutralizing gets channeled against it, hence being regarded a pertinent target antigen in the entire process of vaccine development [13,14].
Conclusion
Scientist started researching on coronavirus vaccines following the outbreaks of MERS and SARS. However, their efforts did succeed due to many challenges. The severe COVID-19 pandemic necessitated urgent efforts to curb the disease since its outbreak was much bigger than the previous incidences of SARS. Moreover, some investigators viewed that the disease could become seasonal and endemic in its occurrence. These reasons coupled with other factors saw many companies and research groups expedite efforts to develop vaccines that would be effective against SARS-CoV-2 globally. The process of COVID-19 vaccine development further led to new technologies and even accelerated the usual stages required to establish and vaccines in humans. The review has shown an essential aspect in the realm of vaccine research, development, and testing of SARS-CoV-2 is depicted by the numerous evaluated technologies, including virus-like particle, nucleic acids (RNA and DNA), recombinant proteins, peptides, inactivated viruses, live attenuated viruses, and viral vector (replicative and non-replicative). Most of these platforms are not presently the basis of other vaccines already in use. Since there is no substantive therapy for serious COVID-19, the development of an effective vaccine coupled with immune enhancing measures become indispensable in fighting this disease. The technology used by different companies and research institutions have successfully generated SARSCoV- 2 vaccines.
References
- Graham SP, McLean RK, Spencer AJ, et al. Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. NPJ vaccines. 2020;5(1):1-6.
- Boopathi S, Poma AB, Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol Structure Dynamics. 2021;39(9):3409-18.
- Rokni M, Ghasemi V, Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: comparison with SARS and MERS. Rev Med Virol. 2020;30(3):e2107.
- Varga Z, Flammer AJ, Steiger P, et al. Electron microscopy of SARS-CoV-2: a challenging task–Authors' reply. The Lancet. 2020;395(10238):e100.
- Covián C, Ríos M, Berríos-Rojas RV, et al. Induction of Trained Immunity by Recombinant Vaccines. Frontiers in Immunol. 2021:3406.
- Xia B, Shen X, He Y, et al. SARS-CoV-2 envelope protein causes acute respiratory distress syndrome (ARDS)-like pathological damages and constitutes an antiviral target. Cell Res. 2021;31(8):847-60.
- Inagaki H, Saito A, Kaneko C, et al. Rapid inactivation of SARS-CoV-2 variants by continuous and intermittent irradiation with a deep-ultraviolet light-emitting diode (DUV-LED) device. Pathogens. 2021;10(6):754.
- Woodruff MC, Ramonell RP, Nguyen DC, et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol. 2020;21(12):1506-16.
- Guevara ML, Persano F, Persano S. Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Frontiers chem. 2020:963.
- Kowarz E, Krutzke L, Reis J, et al.“Vaccine-Induced Covid-19 Mimicry” Syndrome: Splice reactions within the SARS-CoV-2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines.
- Disantara FP. The Large Scale Social Restrictions Policy For Handling The Covid-19 Pandemic. J Pembaruan Hukum. 2020;7(2).
- https://www.wsj.com/articles/inside-the-hunt-for-a-link-between-some-covid-19-vaccines-and-rare-blood-clots-11620898201.
- Dimonte S, Babakir-Mina M, Hama-Soor T, et al. Genetic Variation and Evolution of the 2019 Novel Coronavirus. Public Health Genomics. 2021;24(1-2):54-66.
- Vogel G, Kupferschmidt K. Side effect worry grows for AstraZeneca vaccine.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref