Research Article - Allied Journal of Medical Research (2022) Volume 6, Issue 11
Children with asthma exposed to COVID-19 implies decrease in severity from its baseline: Systematic Review from March 2020- September 2022
Juel Chowdhury1*, Rejoice P. Ravi2
1Physician, Health Protection & Communicable Diseases Division, Ministry of Public Health, Qatar
2Epidemiologist, Health Protection & Communicable Diseases Division, Ministry of Public Health, Qatar
- *Corresponding Author:
- Juel Chowdhury
Physician, Health Protection & Communicable Diseases Division
Ministry of Public Health, Qatar
E-mail: juel2050@gmail.com
Received: 21-Oct-2022, Manuscript No. AAAJMR-22- 78001; Editor assigned: 25-Oct-2022, PreQC No. AAAJMR-22-78001(PQ); Reviewed: 08-Nov-2022, QC No. AAAJMR-22-78001; Revised: 10-Nov-2022, Manuscript No. AAAJMR-22-78001(R) ; Published: 17-Nov-2022, DOI:10.35841/aaajmr-6.11.151
Citation: Marion W. Working for a Philosophical Context for Psychiatry. Allied J Med Res. 2022;6(11):151
Abstract
Introduction: During the COVID-19 pandemic, children have been severely affected by SARSCoV-2 infection which primarily affects the respiratory system. Children with chronic respiratory diseases such as asthma, emphysema, and chronic bronchitis can experience an exacerbation of the preexisting conditions. This systematic review aims to identify the severity of asthma in COVID-19-exposed children aged 4-12 years. Methods: A systematic review of the literature was performed to gain an understanding of children with asthma exposed to COVID-19. Data were extracted from the PubMed database between March 2020 and September 2022. During the COVID-19 pandemic, 169 PubMedpublished articles involving children aged 4-12 years were identified. Children underwent testing for SARS-CoV-2 and positive test results were included in the study. We screened 28 articles out of 169 PubMed articles. Finally, 7 articles out of 28 articles were included in the study. Results: The number of children with asthma hospitalized owing to COVID-19 across the world was 1% to 2.7%. Incidence was 12.8 times less frequent in children than in adults. Increased expression of the angiotensin-converting enzyme-2 gene in the bronchial epithelium of patients with type 2-low or T1-high asthma tended to have higher known risk factors for COVID-19 including hypertension, and lymphopenia. Among school-age children (N=277,285), 1.2% were hospitalized, 0.1% had intensive care unit admissions, and <0.1% died. Of those patients (hospitalized, ICU admissions, and those who died from COVID-19), each had at least one underlying medical condition and 55% of the underlying conditions were accounted for by chronic lung disease including asthma. Conclusion: Childhood asthma outcomes were improved during the COVID-19 pandemic. This could be due to reduced exposure to asthma triggers. Parents, caregivers, and children are advised to continue proper asthma management.
Keywords
Asthma, Children, Healthcare, SARS-CoV-2, Systematic review.
Introduction
Coronavirus disease 2019 (COVID-19) was declared a pandemic on March 11th, 2020 [1]. The World Health Organization (WHO) and Center for Disease Control and Prevention (CDC) have issued health alerts and prevention guidelines for people at increased risk for severe health outcomes and death due to COVID-19 [2,3]. Chronically diseased patients are particularly vulnerable to severe complications of this disease, so need special attention to help prevent increased morbidity and mortality [4]. Asthma is a chronic disease requiring long-term medical monitoring, and individuals with asthma are particularly vulnerable to the changes resulting from COVID-19 infection [5]. Asthma patients may experience decreased access to healthcare due to restrictions on public movements, during lockdowns and diversion of healthcare resources to the care of patients affected by COVID-19 [6].
Patients with asthma are hypothesized to have a high susceptibility to and increased severity of, SARS-CoV-2 infection due to their impaired immune response. The likelihood of respiratory exacerbation increases when infected with respiratory viruses. But only limited evidence has supported this theoretical risk [7]. It was reported that the risk of severe illness and death from COVID-19 was increased for people with severe asthma and those prescribed high-dose Inhaled Corticosteroids (ICS) [8-11]. An online survey of caregivers of children with asthma from 27 countries and 5 continents revealed that during the COVID-19 pandemic, 47% of asthmatic children had limited access to healthcare services and 20% (10%-40%) experienced better disease control [12]. It was also reported that medication adherence among patients with asthma increased during this pandemic [12,13]. Furthermore, lockdown restrictions, school cancellations, and social distancing have had significant implications for movement and play behaviors, limiting children’s Physical Activity (PA) and reducing exposure to environmental triggers [14,15]. These changes in health-seeking behaviors, medication management, and the environment may potentially impact asthma control [15]. With the virus so easily transmitted, effects on the pediatric community were inevitable, and the very first reported case was encountered in January 2020 in a 10-year-old boy who contracted the virus in Wuhan [16]. Since then, a multitude of pediatric cases has been reported globally. To date, the data reveal that the course of the disease is milder in children than in adults, but it is unclear what the role of type-2 inflammation is in the outcome of asthmatic children infected by SARS-CoV-2. Therefore, we performed a systematic review on children with asthma exposed to COVID-19 that showed a decrease in severity from its baseline during the pandemic period.
Methods
Study design and setting
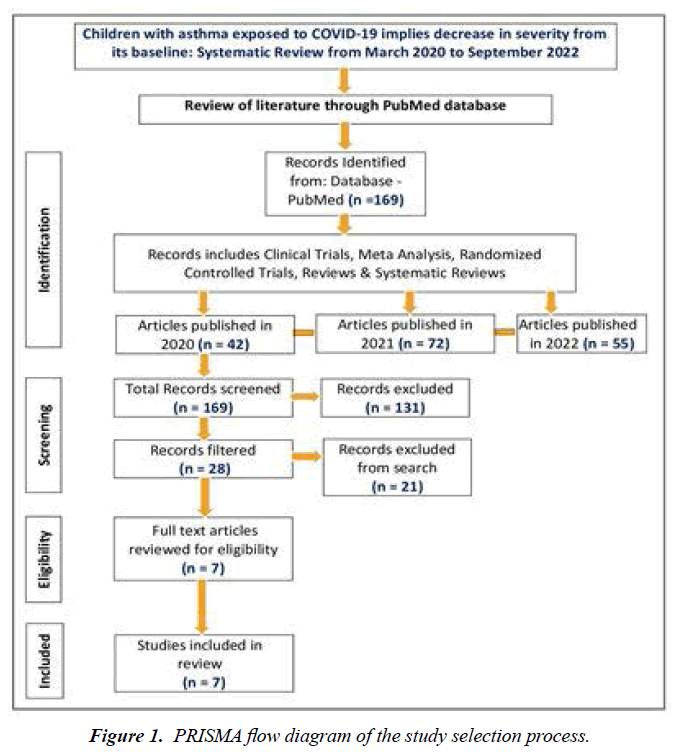
A systematic review of the literature was performed as per the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to identify the severity of asthma in COVID-19-exposed children aged 4-12 years in three phases. In the first phase, we searched and identified 169 PubMed articles, including Clinical Trials, Meta-Analysis, Randomized Controlled Trials, Reviews, and Systematic Reviews. Secondly, we reviewed and screened 28 out of 169 PubMed articles on asthmatic children aged 4-12 years who underwent testing for SARS-CoV-2 and the test results were positive. Finally, we included 7 articles out of 28 full-text articles in the study. Data were extracted from the PubMed database between March 2020 and September 2022. Out of 169 articles, 42 PubMed articles have been published in 2020, 72 articles have been published in 2021 and 55 articles have been published as of September 2022 (Figure 1).
Search terms related to COVID-19 infection and asthma in children aged 4 to 12 years were used. Quality was assessed using the modified Assessment of Multiple Systematic Reviews (AMSTARS) criteria and data were extracted. The inclusion criteria for enrollment were children who had experienced asthma in the age group of 4-12 years. Children who had undergone testing for SARS-CoV-2 tested positive. Children who had comorbidities such as cardiovascular disease, diabetes, cancer, immunosuppression and obesity before COVID-19 infection were excluded. Both hospitalized and community settings were included in the study. Children with asthma: Clinical Asthma Score (CAS) values were grouped into three categories: mild (0-4), moderate (5-8) and severe (9-12) were included [17,18].
Study selection and inclusion criteria
Study types considered were Clinical Trial, Meta-Analysis, Randomized Controlled Trial, Review, and Systematic Review that reported on COVID-19 among children with asthma. We extracted only studies that included a combined report of COVID-19 among children with asthma. We excluded studies that used empirical data that was not original, asthma exacerbations in children, COVID-19 co-morbidities such as diabetes, cancer, immunosuppression and obesity and not confirmed tests for COVID-19. Studies that described or applied a severity score that could be utilized to measure asthma conditions, in patients from 4 to 12 years of age, were included. The “severity score” must have had a numeric value associated with each measured parameter to allow variation in severity to be assessed. Studies that assessed dyspnea in relation to other conditions such as bronchiolitis and rhinitis and articles that used a predictive score (looked at the need for hospitalization) were excluded. The title, the abstract, and the full-text screening process were systematically followed for the final selection of the study. Duplicate records were excluded and misunderstandings regarding the inclusion of this study were resolved through discussions with colleagues in the same department.
Data extraction
The required data for the systematic review were extracted and inconsistencies were solved through discussion with colleagues. Data were gathered on publication date, study type, age group, gender and children with asthma mild (0- 4), moderate (5-8) and severe (9-12), and pediatric asthma exacerbation in children. Data has been extracted for COVID- 19-related clinical symptoms and other comorbidities. The severity of COVID-19 disease was classified as mild (i.e., non-pneumonia and mild pneumonia), severe (i.e., dyspnea, respiratory frequency ≥ 30/min, blood oxygen saturation ≤ 93%, and/or lung infiltrates>50% within 24-48 h) and critical (i.e., respiratory failure, septic shock, and/or multiple organ dysfunction or failure) [19]. Admission to the Intensive Care Unit (ICU) and recovery status (cured, died, still in hospital) was also assessed and reported.
Results
A systematic review of the literature was performed to gain insight into children with asthma exposed to COVID-19 during the pandemic. Data were extracted from the PubMed database between March 2020 and September 2022. We searched and identified 169 PubMed articles. We screened 28 articles out of 169 PubMed articles. Finally, we included 7 articles out of 28 articles in the study.
COVID-19 and prevalence, incidence and risk of asthma among children
The number of children with asthma hospitalized owing to COVID-19 across the world is low, with incidence reported from 1% to 2.7% [20]. An online questionnaire sent to 91 pediatric practitioners in 27 countries attempted to estimate the incidence of clinically relevant COVID-19 in pediatric patients with asthma. They noted that incidence was 12.8 times less frequent in children than in adults [12]. A retrospective study of a large cohort in Israel also showed that patients with asthma have a lower susceptibility to COVID-19 in pediatric and adult patients. The study did not find any difference in the rate of hospitalization in patients with COVID-19 with or without asthma [21]. A nationwide study in Japan examining asthma during the COVID-19 outbreak found decreased asthma admissions in 2020 compared with previous years for children and adults [22]. Similarly, 212 children with allergic asthma in Spain reported no significant difference in asthma control or severity between patients with and without COVID-19 [23]. The early data from Wuhan regarding hospitalized pediatric patients and those with severe COVID-19 do not list asthma as a risk factor [24,25]. The study included 1,054 children with asthma and 505 control subjects, from 25 pediatric departments in 15 countries globally. A higher proportion of males (62.8% vs 51.9%) were observed in the asthma group compared to the controls, which is consistent with epidemiological characteristics of the disease in this age group [26]. A total of 297 parents/caregivers participated and their response rate was 79%. Most children (188 of 63%) were male. Three age groups were defined: 4-6, 7-12, and 13-17 years with 50% aged 7-12 years. The majority, 104 (35%), were diagnosed with asthma between the ages of 6 and 12 years [27]. In another study, 39 (65.0%). Out of 60 patients with asthma were male [28]. Treatment and asthma control data for 475 children were analyzed from 18 May to 15 June 2020. The median age was 5.6 years and 60.6% were males [29].
Effect of COVID-19 pandemic on adherence and asthma control
Various studies from around the world, including China, Brazil, Italy, Switzerland and the United States, reveal that asthma is not associated with an increased risk of mortality in adult patients with COVID-19 [20]. The Morbidity and Mortality Weekly Report from October 2020 that evaluated COVID-19 trends among school-age children (N= 2,77285) noted that 1.2% were hospitalized, 0.1% had Intensive Care Unit (ICU) admissions and less than 0.1% died. Amongst them (hospitalized, ICU admissions and deaths due to COVID-19), each had at least one underlying medical condition, and 55% of the underlying conditions were accounted for by chronic lung disease including asthma and emphysema [30].
Cough, shortness of breath, vomiting and diarrhea was significantly more common in the asthma group than in the control group. Hospitalization was significantly higher in the asthma group and hospitalization duration was significantly longer in the control group. There were no statistically significant differences between the asthmatic and control groups in terms of needing oxygen treatment and laboratory findings [31]. Among respondents, 181(61%) (95% CI 55.1% to 66.5%) reported improved compliance with medications during the lockdown, with the majority of respondents 194(65%) (95% CI 59.6% to 70.7%) reporting ongoing use of preventers at prescribed doses. More than 75% of the children used their bronchodilator <2 days/week 227 (76%) (95% CI 71.2% to 81.1%). Only 23(8%) of the patients required a reliever inhaler ≤ 5 days/week. Around one-third of 103 (35%), parents or caregivers reported reduced controller dosing during the lockdown. The majority 81(79%) indicated this was due to their children’s asthma stability and 22% of parents decreased the dose of inhalers because of fear of drug shortages. A small number mentioned their inability to reach the hospital [27].
During the pandemic, children with asthma experienced fewer upper respiratory tract infections, episodes of pyrexia, emergency visits, hospital admissions, asthma attacks and hospitalizations due to asthma, in comparison with the preceding year. Nearly 66% of asthmatic children had improved asthma control while in 33% the improvement exceeded the minimal clinically relevant difference. Pre-bronchodilatation: Forced Expiratory Volume in 1 second (FEV1) and peak expiratory flow rate were improved during the pandemic. When compared to non-asthmatic controls, children with asthma were not at increased risk of Lower Respiratory Tract Infections (LRTIs), episodes of pyrexia, emergency visits, or hospitalizations during the pandemic. However, an increased risk of Upper Respiratory Tract Infections (URTIs) emerged [26]. According to this study, these 14 children showed a tendency to escalate their treatment (11.8% vs 4.2%), but the difference was not statistically significant. Eleven children (64.7%) received reliever treatment and two (11.8%) required further escalation. Two of these children needed relief and escalation treatment. Seven patients were assisted in the emergency department. Two of these children needed relief and escalation treatment, requiring hospital admission for supplementary oxygen therapy; one of them was admitted to the Pediatric Intensive Care Unit (PICU). Regarding the symptoms they presented, the most frequent was cough/ respiratory distress (41.2%), followed by fever (29.4%), headache (17.6%), gastrointestinal problems (17.6%), sore throat (5.9%) and (35.3%) were asymptomatic. No significant differences were observed in the frequency of symptoms compared to children with negative IgG SARSCoV-2 [20].
Decreased willingness and inability to seek medical care
Nearly all caregivers reported that they were reluctant to seek healthcare if their children had mild asthma symptoms. In addition, they did not visit the asthma clinic regularly, only if symptoms persisted and worsened. The caregivers expressed that they were unwilling to put themselves and their children at higher risk of exposure to the virus and feared being quarantined, which led to delayed hospital visits during the COVID-19 outbreak [32]. Almost one-third (103 (35% of parents/caregivers) reported reduced controller dosing during the lockdown. The majority 81 (79%) indicated this was due to their children’s asthma stability [27].
Use of telemedicine during the COVID-19 lockdown
During the pandemic, due to isolation at home and fear of being admitted to the hospital for diagnosis and treatment, the caregivers chose to access healthcare through virtual online or telephone consultations with professional doctors who had treated their children before and knew their conditions well to enable the continuation of treatment. Of the 64 parents who contacted their child's medical team, 87 percent described it as convenient and easy to access. The child’s medical team was contacted by 76 (26%) at least once. Methods were most commonly WhatsApp 45(152%) and phone 45(15%), with more than one option possible [27]. Nevertheless, several caregivers had some concerns about virtual consultation and regarded it as a suboptimal clinical encounter in which doctors could not comprehensively assess their children’s situations [32]. Most of the parents (91.6%) did not need to be apart from their children during the COVID-19 pandemic period and more than half of the parents (66.6%) seemed to have adapted to the new normal and adjusted their lives to the changing conditions [28].
Discussion
We performed a systematic review in children with asthma exposed to COVID-19 suggesting a decrease in severity from its baseline during the pandemic period. A primary goal of this study was to provide updated evidence that high-risk children require medical care and support from their families when vulnerable groups like those with COVID-19 are infected. For children with asthma to attend school safely, attention should be paid to asthma control, risk stratification and compliance with medication [33]. However, during the pandemic period, the treatment of chronic diseases may be interrupted for various reasons. Parental anxiety is one of them, especially for immunotherapy [34]. One of every five patients with asthma reported that their treatment was disrupted during the pandemic period, and some of them gave up admitting to the hospital due to the risk of COVID-19 despite having complaints [35].
During the pandemic, children with asthma experienced improved disease control (two-thirds of the patients), as evidenced by improved scores in validated asthma control measures, fewer asthma attacks, fewer hospitalizations, and better pulmonary function. The multifaceted etiology of this observation may include the avoidance of major asthma triggers including outdoor allergens, viral infections, physical exercise and air pollution, due to social distancing, home sheltering, and reduced school days [36-41]. It shows that the childhood asthma cohort showed improved asthma activity and health during the COVID-19 pandemic, likely as a result of decreased exposure to asthma triggers and increased adherence to treatment. It also demonstrated that during the pandemic, children with predominantly mild to moderate atopic asthma did not suffer from an increase in the frequency of acute episodes that could represent COVID-19 infection [26].
During the COVID-19 pandemic, children have been greatly affected by the sudden withdrawal from school, social life and outdoor activities. Several studies have shown that prolonged school closure and home confinement negatively affect children's psychological and mental states, cause behavioral problems and increase stress in children [42-44]. It shows that high rates of anxiety and depressive symptoms occur in children and adolescents due to the pandemic itself, social isolation and parental stress [45]. Some children may also have experienced increased domestic violence during this period [46]. It is thought that with adequate and timely intervention, the damage caused by this pandemic to mental health can be reduced [46]. Irregular sleep pattern is one of the most significant factors affecting mental health [47,48]. A study conducted in Italy showed that children have a significant delay in sleep timing and worsening sleep quality during the lockdown due to COVID-19 [49]. It showed that the COVID-19 pandemic affects mental health more in young people with asthma/allergies than those without asthma/ allergies. It was determined that asthmatic patients with changes in their sleep patterns had a higher fear of COVID-19 infection in our study [50].
The majority of caregivers perceived that their children’s level of asthma control was maintained or even improved during the epidemic. The maintenance or improvement of asthma control was probably partly mediated by increased medication adherence, which is critical to optimal asthma control [51], driven by enhanced awareness of asthma control and reduced exposure to asthma triggers [52], most notably respiratory infections [53,54] and outdoor allergens [55], due to the COVID-19 outbreak. These results indicated that even under the pressure of lockdown, children should continue good asthma management. They should adhere to medication treatments and avoid environmental triggers to control and prevent asthma symptoms and attacks [56]. They conducted an online survey in 27 countries and 5 continents and reported that 20% (10%-40%) of children with asthma had improved asthma control with no apparent deterioration compared with that before the epidemic. Medication adherence among patients with asthma during the COVID-19 epidemic reported a 14.5% increase in adherence [57].
Conclusion
The SARS-CoV-2 pandemic has caused severe illness and a high death rate worldwide. Lower rates of COVID-19 disease than had been expected among children. During the COVID-19 lockdown, most of the children with asthma and recurrent wheezing maintained their preventive treatments unchanged, showing effective therapeutic adherence. Therefore, their underlying disease remained well controlled. The severe lockdown due to the COVID-19 pandemic was associated with a significant decrease in Emergency Department presentations and hospitalizations among children with asthma. Among children with asthma, those receiving subcutaneous immunotherapy are the ones whose treatments are most affected. Increased medication adherence driven by enhanced awareness of asthma control and reduced exposure to asthma triggers, most notably respiratory infections and outdoor allergens, due to the COVID-19 outbreak might result in improved asthma control.
Ethical Approval
Ethical approval was not necessary because this study was performed using data from published articles in PubMed by the National Center for Biotechnology Information (NCBI).
Availability of Data and Materials
All data related to this systematic review are from published articles in the databases of PubMed, NCBI, National Library of Medicine (NLM) and the National Institute of Health (NIH).
Competing Interest
The authors declare that they have no competing interests.
Funding
Not applicable
Authors’ Contributions
Juel Chowdhury and Rejoice Puthuchira Ravi contributed to the concept, drafting and reporting of the systematic review. JC contributed to the revision of the manuscript. All authors have read and approved the final manuscript.
Conflict of Interest
The authors have no conflict of interest.
Contributions
Juel Chowdhury: Drafting of the manuscript for content, including medical writing for content, screening and scrutinizing and selecting the data, flowchart preparation and interpreting the data.
Rejoice Puthuchira Ravi: Search and identify the articles, screening and selecting the articles, drafting of the manuscript for content, preparation of flowchart and interpreting the data.
References
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. NEJM. 2020.
- CDC. coronavirus disease 2019 (COVID-19). https://www.cdc. gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. 2020.
- WHO. Coronavirus disease (COVID-19) pandemic. https://www. who.int/emergencies/diseases/novel-coronavirus-2019. 2020.
- Saqib MA, Siddiqui S, Qasim M, et al. Effect of COVID-19 lockdown on patients with chronic diseases. Diabetes Metab Syndr Clin Res Rev. 2020;14(6):1621-3.
- Oreskovic NM, Kinane TB, Aryee E, et al. The unexpected risks of COVID-19 on asthma control in children. J Allergy Clin Immunol Pract. 2020;8(8):2489-91.
- Chudasama YV, Gillies CL, Zaccardi F, et al. Impact of COVID-19 on routine care for chronic diseases: a global survey of views from healthcare professionals. Diabetes Metab Syndr Clin Res Rev 2020;14(5):965-7.
- Liu S, Zhi Y, Ying S. COVID-19 and asthma: reflection during the pandemic. Clin Rev Allergy Immunol. 2020;59(1):78-88.
- National Institute for Clinical Excellence. COVID-19 rapid guideline: severe asthma, 2020. Available: https://www.nice.org.uk/guidance/ ng166
- Bloom CI, Drake TM, Docherty AB, et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med. 2021;9(7):699-711.
- Schultze A, Walker AJ, MacKenna B, et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med 2020;8(11):1106-20.
- Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430-6.
- Papadopoulos NG, Custovic A, Deschildre A, et al. Impact of COVID-19 on pediatric asthma: practice adjustments and disease burden. J Allergy Clin Immunol Pract. 2020;8(8):2592-9.
- Kaye L, Theye B, Smeenk I, et al. Changes in medication adherence among patients with asthma and COPD during the COVID-19 pandemic. J Allergy Clin Immunol Pract 2020;8(7):2384.
- Moore SA, Faulkner G, Rhodes RE, et al. Impact of the COVID-19 virus outbreak on movement and play behaviours of Canadian children and youth: a national survey. IJBNPA. 2020;17(1):1-1.
- Oreskovic NM, Kinane TB, Aryee E, et al. The unexpected risks of COVID-19 on asthma control in children. J Allergy Clin Immunol Pract . 2020;8(8):2489-91.
- Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The lancet. 2020;395(10223):514-23.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-42.
- Parkin PC, Macarthur C, Saunders NR, et al. Development of a clinical asthma score for use in hospitalized children between 1 and 5 years of age. CEGH. 1996;49(8):821-5.
- Liu LL, Gallaher MM, Davis RL, et al. Use of a respiratory clinical score among different providers. Pediatr. Pulmonol. 2004;37(3):243-8.
- Skevaki C, Karsonova A, Karaulov A, et al. Asthma-associated risk for COVID-19 development. Journal of Allergy and Clinical Immunology. 2020;146(6):1295-301.
- Green I, Merzon E, Vinker S, et al. COVID-19 susceptibility in bronchial asthma. J Allergy Clin Immunol Pract . 2021;9(2):684-92.
- Abe K, Miyawaki A, Nakamura M, et al. Trends in hospitalizations for asthma during the COVID-19 outbreak in Japan. J Allergy Clin Immunol Pract . 2021;9(1):494-6.
- Ruano FJ, Álvarez ML, Haroun-Díaz E, et al. Impact of the COVID-19 pandemic in children with allergic asthma. J Allergy Clin Immunol Pract . 2020;8(9):3172-4.
- Zheng F, Liao C, Fan QH, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci 2020;40(2):275-80.
- Sun D, Li H, Lu XX, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Clin Pediatr. 2020;16(3):251-9.
- Papadopoulos NG, Mathioudakis AG, Custovic A, et al. Childhood asthma outcomes during the COVID?19 pandemic: findings from the PeARL multi?national cohort. Allergy. 2021;76(6):1765-75.
- Al-Iede M, Waters K, Aleidi SM, et al. Impact of COVID-19 lockdown on children with asthma in Jordan: a parental questionnaire. BMJ Paediatr Open. 2021;5(1).
- Hepkaya E, Kilinc AA, Cebi MN, et al. General health status of children with asthma during the COVID?19 pandemic. Pediatr Int. 2021;63(3):331-7.
- Daoud Pérez Z, Rázquin Arias M, López?Escobar A, et al. The impact of COVID?19 lockdown on children with recurrent wheezing and asthma in Spain. J Paediatr Child Health. 2022;58(9):1635-41.
- Leeb RT, Price S, Sliwa S, et al. COVID-19 trends among school-aged children United States, March 1–September 19, 2020. MMWR. 2020;69(39):1410.
- Metbulut AP, Mustafao?lu Ö, ?en G, et al. Evaluation of the clinical and laboratory findings of asthmatic children with SARS-CoV-2 infection. Int Arch Allergy Immunol. 2021;182(10):989-96.
- Jia Y, Bao J, Yi M, et al. Impact of the COVID-19 pandemic on asthma control among children: a qualitative study from caregivers’ perspectives and experiences. BMJ open. 2021;11(5):e046525.
- Boechat JL, Wandalsen GF, Kuschnir FC, et al. COVID-19 and pediatric asthma: clinical and management challenges. IJERPH. 2021;18(3):1093.
- Celik IK, Metbulut AP, Uneri OS, et al. Effect of patient and parental anxiety on adherence to subcutaneous allergen immunotherapy during the coronavirus disease 2019 pandemic. Ann. Allergy Asthma Immunol. 2021;126(5):595-7.
- Cekic S, Karali Z, Cicek F, et al. The Impact of the COVID-19 Pandemic in Adolescents with Asthma. JKMS. 2021;36(49).
- Wu H, Zhu H, Yuan C, et al. Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3(6):e2010895-.
- Krivec U, Seliger AK, Tursic J. COVID-19 lockdown dropped the rate of paediatric asthma admissions. Arch Dis Childh. 2020;105(8):809-10.
- Niespodziana K, Borochova K, Pazderova P, et al. Toward personalization of asthma treatment according to trigger factors. J. Allergy Clin Immunol. 2020;145(6):1529-34.
- Eguiluz?Gracia I, Mathioudakis AG, Bartel S, et al. The need for clean air: the way air pollution and climate change affect allergic rhinitis and asthma. Allergy. 2020;75(9):2170-84.
- Taquechel K, Diwadkar AR, Sayed S, et al. Pediatric asthma health care utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract . 2020;8(10):3378-87.
- Chavasse R, Almario A, Christopher A, et al. The indirect impact of COVID-19 on children with asthma. Arch Bronconeumol. 2020;56(11):768.
- Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. The lancet. 2020;395(10227):912-20.
- Jiao WY, Wang LN, Liu J, et al. Behavioral and emotional disorders in children during the COVID-19 epidemic. J Pediatr. 2020;221:264-6.
- Sprang G, Silman M. Posttraumatic stress disorder in parents and youth after health-related disasters. Disaster Med Public Health Prep. 2013;7(1):105-10.
- Saraçlar Y, Kuyucu S, Tuncer A, et al. Prevalence of asthmatic phenotypes and bronchial hyperresponsiveness in Turkish schoolchildren: an International Study of Asthma and Allergies in Childhood (ISAAC) phase 2 study. Ann Allergy Asthma Immunol. 2003;91(5):477-84.
- Celik IK, Metbulut AP, et al. Effect of patient and parental anxiety on adherence to subcutaneous allergen immunotherapy during the coronavirus disease 2019 pandemic. Ann. Allergy Asthma Immunol. 2021;126(5):595-7.
- de Figueiredo CS, Sandre PC, Portugal LC, et al. COVID-19 pandemic impact on children and adolescents' mental health: Biological, environmental, and social factors. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110171.
- Mindell JA, Williamson AA. Benefits of a bedtime routine in young children: Sleep, development, and beyond. Sleep Med Rev. 2018;40:93-108.
- Cellini N, Di Giorgio E, Mioni G, et al. Sleep and psychological difficulties in Italian school-age children during COVID-19 lockdown. J Pediatr Psychol. 201;46(2):153-67.
- Hawke LD, Monga S, Korczak D, et al. Impacts of the COVID?19 pandemic on youth mental health among youth with physical health challenges. Early Interv Psychiatry. 2021;15(5):1146-53.
- Horne R, Price D, Cleland J, et al. Can asthma control be improved by understanding the patient's perspective? BMC Pulm. Med. 2007;7(1):1-1.
- Gautier C, Charpin D. Environmental triggers and avoidance in the management of asthma. Journal of asthma and allergy J Asthma Allergy. 2017;10:47.
- Pelaia G, Vatrella A, Gallelli L, et al. Respiratory infections and asthma. Respir Med. 2006;100(5):775-84.
- Nnodum BN, McCormack MC, Putcha N, et al. Impact of physical activity on reporting of childhood asthma symptoms. Lung. 2017;195(6):693-8.
- Dales R, Chen L, Frescura AM, et al. Acute effects of outdoor air pollution on forced expiratory volume in 1 s: a panel study of schoolchildren with asthma. Eur Clin Respir J. 2009;34(2):316-23.
- Papadopoulos NG, Custovic A, Deschildre A, et al. Impact of COVID-19 on pediatric asthma: practice adjustments and disease burden. J Allergy Clin Immunol Pract . 2020;8(8):2592-9.
- Kaye L, Theye B, Smeenk I, et al. Changes in medication adherence among patients with asthma and COPD during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(7):2384.
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref
Indexed at, Google scholar, Cross Ref