Case Report - Current Trends in Cardiology (2020) Volume 4, Issue 1
Case report: A young man with a rare grown-up congenital heart disease.
Angel D Cueva1*, Giancarlo A Valle1, Félix A Revilla1, Sara Ramírez-Flores2, Aureo Campos-Tello1
1Department of Cardiology, Hospital Nacional Dos de Mayo, Lima, Peru
2Department of Cardiology, Hospital Central FAP, Miraflores 15046, Peru
- Corresponding Author:
- Angel D Cueva
Department of Cardiology of Hospital Nacional Dos de Mayo
Lima
Peru
E-mail: afernandocampos@gmail.com
Accepted date: June 29, 2019
Citation: Cueva AD, Valle A, Revilla A , et al. Case report: A young man with a rare grown-up congenital heart disease. Curr Trend Cardiol 2020; 4(1):1-4.
Keywords
Pulmonary Artery, Pulmonary Circulation, Valve Stenosis.
Introduction
Truncus arteriosus (TA) is a rare, congenital heart defect characterized by a single great artery giving rise to the ascending aorta, pulmonary arteries, and coronary arteries. TA ocurred in 0.21%-0.34% of all cases of congenital heart disease and presents with a right-sided aortic arch or an interrupted aortic arch (IAA) in 18-36% and 11-14% of cases, respectively [1].
TA is classified using the Van Praagh and Collette and Edwards classification systems, depending on the origins of the pulmonary arteries [2,3]. Collett and Edwards based their system only on the origins of the pulmonary vascular system, while Van Praagh also took into account aortic abnormalities [4].
In this condition, the systemic circulation, pulmonary circulation, and coronary circulation receive a mixture of oxygenated and deoxygenated blood; therefore, cyanosis may be seen in early postnatal life. The presentation includes, as well, congestive cardiac failure within few weeks of life and commonly present with complaints of dyspnea with feeding and failure to thrive [5].
We report a case of a TA with ventricular septal defect, unique coronary artery and acquired double mitral lesion. This case is important because almost no patient with TA can reach young age, even with an acquired valvular pathology.
Case Presentation
A 17 year old male from Iquitos (a far region in our country), diagnosed with ventricular septal defect (VSD) at the age of two and unsuccessfully referred to Lima at the age of five due to dyspnea and cyanosis, attended to his local hospital for this symptoms and received Losartan 50 mg BID, Digoxin 0.25 mg QD and Spironolactone 25 mg QD. Due to lack of improvement (the previous year he was in NYHA II functional class, and then he progressed to NYHA III functional class despite treatment) he came to a consult in our department. At physical exam, the patient presented signs of clubbing and systemic congestion. His vitals were: heart rate of 85 bpm, BP of 100/60 mmHg, tachypnea with 22 breath/minute and blood oxygen saturation of 79%. She had left ventricular impulse displaced to the left, a diastolic murmur in the aortic accessory area, another diastolic murmur in the mitral area and bibasal crackles. He was given Furosemide 20 mg IV TID, Enalapril 2.5 mg PO BID and Spironolactone 25 mg PO QD in the acute phase. When compensated, the treatment was the following: Furosemide 40 mg PO BID, Enalapril 5 mg PO BID, Bisoprolol 2.5 mg PO QD and Spironolactone 25 mg PO QD.
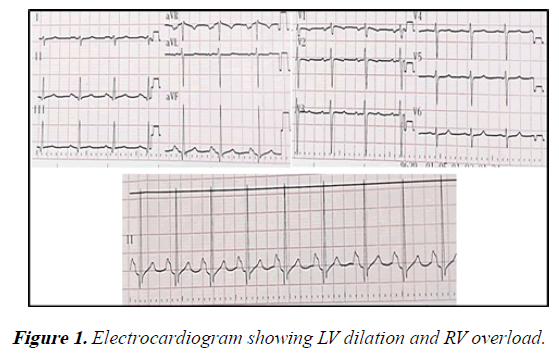
Electrocardiography demonstrated sinus rhythm, axis deviated to the right (100°), signs of left ventricular dilation, right ventricle overload and dilation of both atria (Figure 1).
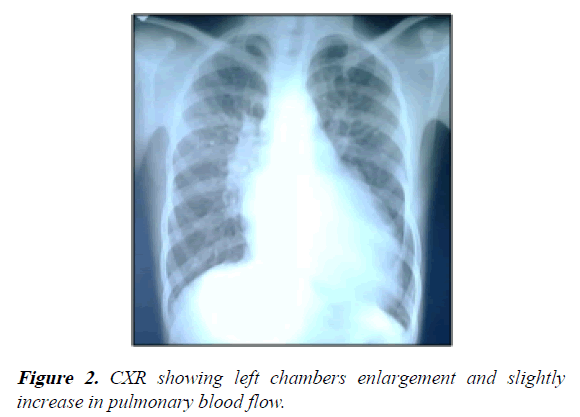
Chest X-ray showed a severe enlargement of the cardiac silhouette (LV and LA dilation) with slightly increased pulmonary flow (Figure 2).
The transthoracic echocardiography showed the following findings:
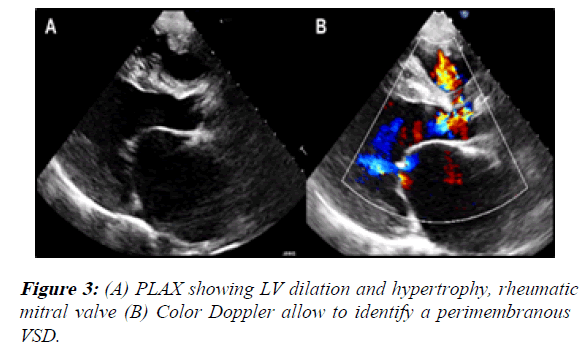
1) The PLAX view showed LV dilation with a telediastolic diameter of 59.8 mm (46.6 mm/m2 indexed to BSA) and a telesystolic diameter of 43.1 mm (33 mm/m2 indexed to BSA). Also, both ventricles had hypertrophy. The LV ejection fraction was 50%. We also identified a membranous VSD with left to right shunt (it measured 20 × 11 mm) (Figure 3).
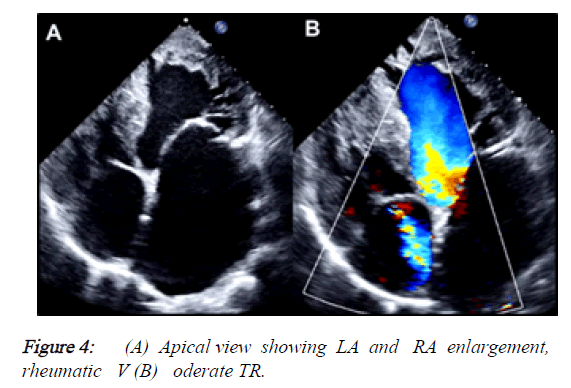
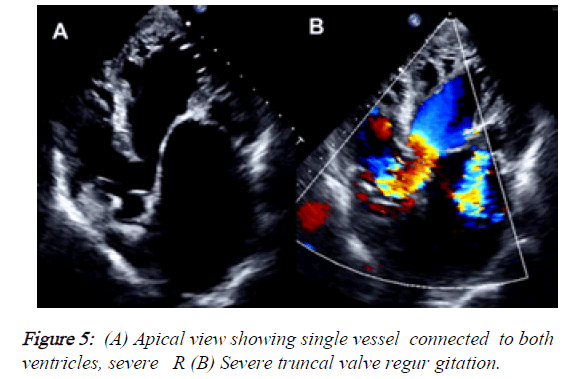
2) Besides, the PLAX view demonstrated that the mitral valve had reduced opening due to rheumatic changes, provoking a severe mitral stenosis (MVA: 0.83 cm2, mean gradient: 12 mmHg) but also had severe regurgitation with a central jet reaching the LA roof. The tricuspid valve also had moderate regurgitation (Vmax: 3.27 m/s, peak gradient: 43 mmHg). The estimated systolic pressure of PA was: 58 mmHg. LA was severely dilated and RA had moderate enlargement (Figures 4 and 5).
3) In the apical view it was notorious that there was a single great vessel connected to both ventricles. The alleged aortic valve was sclerotic and had a jet of severe regurgitation. In the PSAX view, we couldn ’ t find the pulmonary trunk or its branches.
The CT showed these findings:
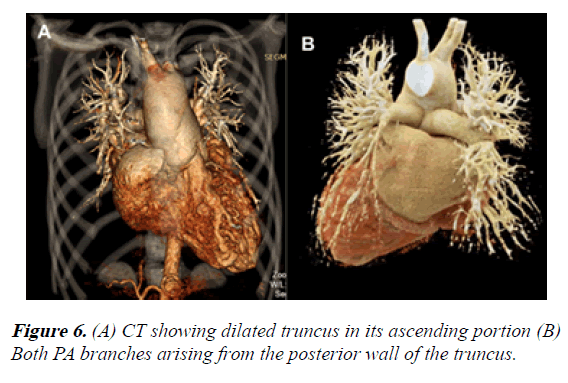
1) Dilation of the truncus in its ascending portion, even affecting the aortic arch. The aortic arch was left sided
2) Both PA branches arising from the posterior wall of the single artery. They showed appropriate development (Figure 6).
3) The VSD measured 25 × 13.3 mm.
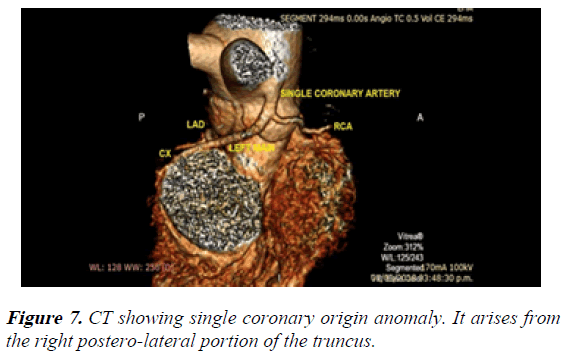
4) Single coronary artery arising from the posterior wall of the truncus. The LMCA and the RCA emerged from that single coronary artery (Figure 7).
With all that information we concluded as follows: type II of Collett-Edwards classification or A2 Van Praagh classification truncus arteriosus, single coronary artery anomaly and rheumatic double mitral lesion.
We evaluated the functional class with a 6 MWT; he walked just 240 meters (less than 50% of the predicted for his age). Unfortunately, the patient’s mother requested for voluntary discharge (because they had few education considering that they lived far away in a small province), and 4 weeks later he died suddenly in unknown circumstances in his city. That’s why we couldn’t perform cardiac catheterization.
Discussion
Truncus arteriosus (TA) accounts for 0.3% of all CHD [1]. Without surgical intervention, most patients would die before their first year of life. However, with corrective surgery longterm functional class of patients is good, despite the high reoperation rates [6]. Collett and Edwards, using anatomic criteria, classified the TA into 4 types: type I: aorta and pulmonary artery share a common <arterial trunk, type II: right and left pulmonary arteries arise separately from the posterior part of the truncus, type III: separate origins of the pulmonary arteries from the lateral aspect of the truncus, type IV: none of the branches arise from the common trunk with the lungs supplied by collaterals [3].
On the other hand, Van Praagh et al., using embryological criteria, classified truncus in type A (with VSD) and type B (without VSD) and its subtypes: type A1: aorta and pulmonary artery arise from the root of a common trunk; this corresponds to type I according to Collett and Edwards classification, type A2: there is absence of the aorto-pulmonary septum, and thus RPA and LPA arise from the truncus arteriosus; this corresponds to type II according Collett and Edwards classification type A3: one branch of PA (usually the right) arises from the common trunk, with other lung supplied either by collaterals or a pulmonary artery arising from the aortic arch, type A4: the aortic arch is either hypoplastic or interrupted, and there is a large PDA. [2]. A frequent associated defect is a VSD which allows the formation of a single outflow tract for both ventricles. There are another associated anomalies like: aortic arch interrupted, PDA, truncal valve abnormalities (bicuspid and quadricuspid with regurgitation or stenosis) and anomalous coronary origin [3].
Few cases of TA in young or adults are reported in the literature. Moreover, the majority of cases are type I TA. For example, Mittal et al described a type I in a 16-year-old male with cyanosis complaining of shortness of breath and fatigue on exertion [7]. Venkatraman D et al. reported a case a 18-yearold female with poor exercise tolerance and recurrent respiratory tract infection and cyanosis who later was diagnosed with type I TA. The above cases were from India [8]. Espinola et al. reported a case series of six patients in Mexico with TA, all of them with type I [9]. As noted, all cases are reported in countries whose health’ s systems failed to identify this disease during the newborn or infancy period, and the case reported in this paper met this pattern.
Our case becomes interesting because it’s not also a type II (A2) truncus arteriosus with coronary anomaly but it is combined with an acquired condition like rheumatic double mitral lesion.
The question that must be addressed is: how could a patient like this one reached 17 years old of age?
The cases previously described found some features that could increase the life expectancy in these type of patients as: truncal valve moderate to severe stenosis, type I TA and pulmonary branches atresia or hypoplasia. All of them share something in common: they limit pulmonary blood flow. It seems that this is the main feature that can make TA patients survive until young or adult age [7-9].
However, our patient didn’t have those characteristics. In fact, he had type II (A2) TA without truncal valve stenosis but with regurgitation. Usually, this pattern behaves as a CHD with increased pulmonary blood flow that makes difficult these patients survive beyond the newborn or infancy period. We think, that in this case, the acquisition of a rheumatic valve stenosis and regurgitation developing pulmonary hypertension “protected” this patient from suffering the consequences of increased pulmonary blood flow. Then, he started to have symptoms as dyspnea and shortness of breath due to progressive decrease in systolic function (LVEF: 50%) of a dilated LV which is quite common in conditions of volume overload (severe regurgitation of the truncal valve).
We are conscious that it ’ s extremely important to assess pressures in pulmonary bed because it helps in the management and is prognostic. Nevertheless, due to voluntary discharge requested by patient’s family it was not possible to perform cardiac catheterization. This is the main limitation of this case report.
Conclusion
We decided to publish this case because it’s rare a type II (A2) TA to survive until young age, so infrequent that we couldn’t find similar case report in current literature. Also, the concomitant occurrence of rheumatic mitral disease makes this patient still more interesting. In fact, we consider the latter to be one of the factors, that possibly, contributed to increase life expectancy of this patient.
References
- Mavroudis C, Backer CL(2013) Truncus arteriosus. In: Mavroudis C, Backer CL (eds) Pediatric cardiac surgery. (4th ed), Wiley-Blackwell, Chichester, West Sussex, UK, Wiley-Blackwell. pp: 361-375.
- Van Praagh R, Van Praagh S. The anatomy of common aorticopulmonary trunk (truncus areteriosus communis) and its embryologic implications. A study of 57 necropsy cases. Am J Cardiol. 1965;16(3):406–425.
- Collett RW, Edwards JE. Persistent truncus arteriosus: A classification according to anatomic types. Surg Clin North Am. 1949; 29(4):1245–1269.
- Van Praagh R. Truncus arteriosus: what is it really and how should it be classified? Eur J Cardiothorac Surg. 1987;1(2):65-70.
- Scholz TD, Reinking BE(2011) Congenital heart disease. In: Gleason CA, Devaskar S (eds) Avery's Diseases of the Newborn. (9th ed), Saunders Elsevier, Ch. 55. Philadelphia, PA.
- Naimo PS, Fricke TA, Lee MGY, et al. Long-term outcomes following repair of truncus arteriosus and interrupted aortic arch. Eur J Cardiothorac Surg. 2020; 57( 2):366–372
- Mittal SK, Mangal Y, Kumar S Yadav RR. Truncus arteriosus type 1: A case report. Ind J Radiol Imag. 2006; 16(2);229-31.
- Venkatraman D, Bhandari M, Mukkhopadhyay T, et al. A rare form of presentation of truncus arteriosus in adult. Nig J Cardiol. 2015; 12(2):148-150.
- Espinola-Zavaleta N, Muñoz-Castellanos L, González-Flores R, et al. Tronco arterioso común en adultos. Arch Cardiol Mex.2008; 78(2):210-216.