Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2021) Volume 11, Issue 83
Analysis on the sample types compatible with different types of SARSCoV-2 diagnostic test
Wenfa Ng*
Department of Biomedical Engineering, National University of Singapore, Lower Kent Ridge, Singapore
- Corresponding Author:
- Wenfa Ng
Department of Biomedical Engineering
National University of Singapore
Lower Kent Ridge
Singapore
E-mail: ngwenfa771@hotmail.com
Accepted date: October 06, 2021
Abstract
Sample type is the interface that diagnostic test use to peek into disease state of the human body. Usually at the site where illness occur, different types of samples at the same site such as the respiratory system could still be obtained given improvement in technology and medical techniques. But, diagnostic test is typically antagonistic to large number of different types of samples given the higher cost and analytical complexity associated with developing a diagnostic test compatible with many types of sample. Hence, diagnostic tests such as those for COVID-19 surveillance could only work with a small number of sample type. This work conducts a meta-analysis of a subset of SARSCoV- 2 diagnostic tests approved for provisional use in Singapore, with particular focus on the types of samples compatible with each category of test. Reverse transcriptase quantitative polymerase chain reaction (RT-qPCR), antigen rapid test, and antibody test are profiled in this work. Results revealed that RT-qPCR test could be used with a larger variety of samples probably due to its gold standard status for detecting COVID-19. But, most RT-qPCR test could only work with nasopharyngeal, oropharyngeal and nasal swab sample. Such samples present discomfort to the individual and is a challenge to implementing an active COVID-19 surveillance programme. Similarly, antigen rapid test sample the nasopharyngeal, oropharyngeal and nasal regions of the respiratory tract, which in presenting discomfort to the individual, also cause challenge to implementing an antigen rapid testbased active COVID-19 surveillance programme. Finally, antibody-based test mainly relies on serum and plasma samples, which meant that sample processing is still a key part of the analytical workflow. However, there is an emerging trend in using whole blood for diagnostic purposes in antibody test. Overall, type of samples compatible with different categories of SARS-CoV-2 test remain limited due to cost pressure and issues with analytical complexity for tests compatible with multiple types of samples. But, more types of samples are beginning to the used for different categories of COVID-19 diagnostic test, which in reducing the discomfort of the test, also expands our capability for early detection, which is key to controlling the spread of the virus.
Keywords
SARS-CoV-2, Diagnostic test, Type of sample, Nasopharyngeal, Oropharyngeal.
Introduction
Sample type is perhaps the most important parameter which could determine whether a diagnostic test could detect a particular pathogen effectively and efficiently in a timely manner. This comes about due to the presence of potential inhibitors or enzymes in the different types of samples that could interfere with the biochemical or physiochemical mechanisms operative in the diagnostic test [1]. Hence, development of diagnostic test is usually centred on a list of pre-determined sample type that needs to be profiled for a particular diagnostic task. For example, the current set of diagnostic tests for SARS-CoV-2 are typically designed for nasopharyngeal or nasal swab samples [2,3] and could not handle saliva or throat swab sample [4,5].
This work attempts to provide a meta-analysis of the types of samples that could be used for different analytical modalities that profile for the aetiological agent of the current pandemic, SARS-CoV-2. Specifically, sample types that work with RTqPCR, antigen rapid test and antibody (serological) tests are profiled. Data comes from a subset of tests which gained provisional use authorisation from the Health Sciences Authority in Singapore.
Results revealed that most RT-qPCR tests could work with nasopharyngeal, oropharyngeal and nasal swab sample. Given that RT-qPCR is the most sensitive and gold standard test for detecting SARS-CoV-2 [6] the discomfort associated with obtaining samples from the nasopharyngeal and oropharyngeal regions meant that there remains significant resistance to voluntary testing for COVID-19 surveillance effort. This then calls for the development of more RT-qPCR test kits that could handle nasal swab samples. Similarly, the most common types of samples compatible with available antigen rapid test remains those of nasopharyngeal and oropharyngeal, which presents challenges of sampling discomfort for patients. But, room exists for the further development of antigen rapid test compatible with nasal swab sample. Finally, sample types compatible with antibody tests are serum and plasma, which meant that sample processing is needed prior to analytical test for SARS-CoV-2. This unfortunately add to the time needed for the test. But, there are antibody test able to use whole blood as sample type, and this helps significantly cut down the total analytical time needed from sample to result.
Materials and Methods
Information about the various RT-qPCR, antigen rapid test and antibody tests were obtained from a Health Science Authority of Singapore website detailing the tests which have gained provisional use authorisation in Singapore. The submitted documentation for each test was manually analysed to obtain information about the types of samples compatible with the test. Aggregated across different approved tests for each category are the summarized results of compatible sample types for each category of test that is presented in this manuscript.
Results and Discussion
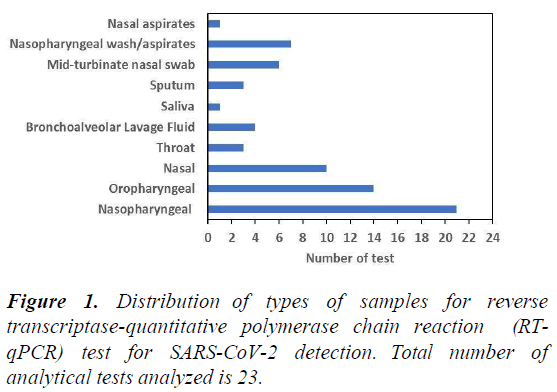
Figure 1 shows the distribution of types of samples for reverse transcriptase quantitative polymerase chain reaction (RTqPCR) test for SARS-CoV-2 detection. The data shows that the most common type of samples that could be used for RT-qPCR are nasopharyngeal, oropharyngeal and nasal swab. Given that these sample types need a swab to be done at the back of the nose (nasopharyngeal) or throat (oropharyngeal), acceptability of these tests for voluntary COVID-19 surveillance is poor, which presents obstacles for active COVID-19 surveillance programmes. Overall, the types of samples that can be used with RTqPCR is large, but most tests rely on nasopharyngeal, oropharyngeal and nasal swab samples.
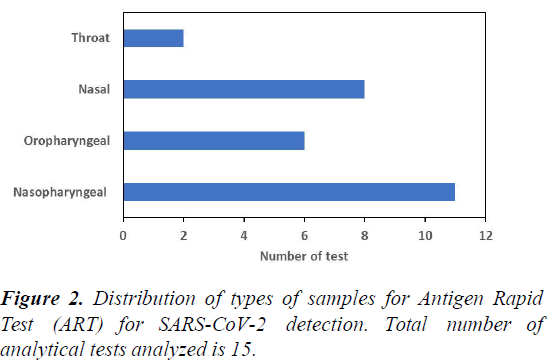
Figure 2 shows the distribution of types of samples for Antigen Rapid Test (ART) for SARS-CoV-2 detection. Results reveal that most ART test rely on nasal swab sample of the nasopharyngeal, oropharyngeal and nasal swab. Similar to the case for RT-qPCR, these sample types need to sample uncomfortable region of the respiratory system, and may present challenges to implementing a voluntary COVID-19 active surveillance programme. But, there is a substantial number of ART test able to use nasal swab samples as input. This sample type is easier to obtain, and does not cause too much discomfort to the individual during sampling, and may be more widely adopted for sampling in ART test in future.
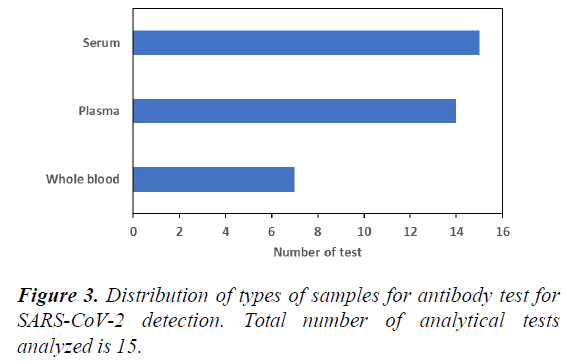
Figure 3 shows distribution of types of samples for antibody test for SARS-CoV-2 detection. Results reveal that most antibody tests can only accommodate serum or plasma samples, which means that sample processing steps remain needed in the analytical workflow. This add time to the workflow and thus, antibody test at present is not suitable for mass deployment at point-of-care. However, there is new technology that allow the use of whole blood as sample for antibody test. Whole blood sample reduces the need for sample processing, which reduces analytical time.
Conclusion
Type of sample is the connection between diagnostic test and the human body. In essence, it is the interface that allows a peek into a disease state of the human body. Naturally, analytical or diagnostic test should be compatible with as many types of samples as possible. But, in reality, cost pressure and analytical challenges meant that most diagnostic tests are only compatible with one or two sample type. This helps reduce the difficulty of diagnostic test development, thereby, reducing development time and time to market. In addition, focus on one or two sample type helps reduce assay complexity and increases diagnostic test robustness.
This work reveals the result of a meta-analysis of SARS-CoV-2 diagnostic tests approved for provisional use in Singapore. Results revealed that RT-qPCR is compatible with most types of samples. But the preponderance of tests still relies on nasopharyngeal, oropharyngeal or nasal swab sample. These sample types sample an uncomfortable region of the respiratory system, and presents serious challenges to the successful implementation of any active COVID-19 surveillance programme. Similar to RT-qPCR test, antigen rapid test also relies on sampling the nasopharyngeal, oropharyngeal and nasal region of the respiratory system, which, again cause discomfort, and is a serious problem for a voluntary COVID-19 active surveillance programme. Finally, antibody (serological) test mainly relies on serum and plasma samples, but there is an emerging trend in which whole blood sample could be used. Use of whole blood sample reduces the need for sample processing and cuts analytical time for antibody test. Overall, type of samples suitable for different categories of SARS-CoV-2 test remain limited given the need to reduce cost, and development time for different diagnostic tests. However, new types of samples that do not cause serious discomfort during sampling are on the horizon, and may be a gamechanger as we move into the uncertain future combating a virus that remains susceptible to mutation and evolution.
Conflicts of Interest
The author declares no conflicts of interest.
Funding
No funding was used in this work.
References
- Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert Rev Mol Diagn. 2020; 20(5): 453–454.
- Péré H, Podglajen I, Wack M, et al. Nasal Swab Sampling for SARS-CoV-2: A Convenient Alternative in Times of Nasopharyngeal Swab Shortage. J Clin Microbiol. 2020; 58(6): e00721-20.
- Zhou Y, O’Leary TJ. Relative sensitivity of anterior nares and nasopharyngeal swabs for initial detection of SARS-CoV-2 in ambulatory patients: Rapid review and metaanalysis. Plos One. 2021; 16(7): e0254559.
- Miranda-Ortiz H, Fernández-Figueroa EA, Ruíz-García EB, et al. Development of an alternative saliva test for diagnosis of SARSCoV-2 using TRIzol: Adapting to countries with lower incomes looking for a large-scale detection program. Plos One. 2021; 16(8): e0255807.
- Tsang NNY, So HC, Ip DKM. Is oropharyngeal sampling a reliable test to detect SARS-CoV-2? – Authors’ reply. Lancet Infect Dis. 2021; 21(10): 1348–1349 (2021).
- Kevadiya BD, Machhi J, Herskovitz J, et al. Diagnostics for SARS-CoV-2 infections. Nat Mater. 2021; 20(5): 593–605.