Research Article - International Journal of Pure and Applied Zoology (2021) Volume 9, Issue 5
A NEW TORQUAQRATORID ACORN WORM (HEMICHORDATA, ENTEROPNEUSTA) AND ITS ACOELOMORPH ASSOCIATE FROM THE ABYSSAL GULF OF CALIFORNIA, MEXICO
Nicholas D. Holland*
Department of Marine Biology Research, Scripps Institution of Oceanography, University of California at San Diego, La Jolla, CA 92093, United States
- Corresponding Author:
- Nicholas D. Holland
Department of Marine Biology Research, Scripps Institution of Oceanography, University of California at San Diego
La Jolla, CA 92093
United States
E-mail: nholland@ucsd.edu
Received 6th August, 2021; Accepted 25th August, 2021; Published 31th August, 2021
Abstract
During the last fifteen years, the known diversity of acorn worms (enteropneust hemichordates) increased conspicuously with the recognition of a fourth family, the Torquaratoridae. Representatives of the new family typically crawl on the surface of the deep-sea floor in contrast to other acorn worms, most of which inhabit burrows in relatively shallow water. Because the deep ocean is so vast and difficult to sample, torquaratorid diversity will only become evident by exploring previously unsampled regions-one of these is the abyssal Gulf of California (Mexico). The present study describes the most abundant torquaratorid species found living on the deep-sea floor in the southern Gulf of California. By means of a remote operating vehicle, worms were video-recorded alive at depth and then collected by suction sampler and brought to the surface. On the basis of both morphological and molecular data, the new acorn worm species belongs to the previously described genus Yoda (the only acorn worm genus known to be hermaphroditic) and has been named Yoda osborni n. sp. An unexpected additional discovery was that the new acorn worm species is associated with acoelomorph worms (likely commensals) living in the pharyngeal lumen. These acoelomorphs are described here as Thalassoanaperus abyssalis n. sp.; they are noteworthy because almost all of their relatives in the clade Convolutidae are free-living and inhabit shallow depths.Keywords
Deep-sea, Hemichordate, Enteropneust, Acoelomorph, Commensalism
Introduction
Phylum Hemichordata (Bateson et al., 1885) consists of two classes: Enteropneusta (Gegenbaur, 1870) (commonly called acorn worms) and Pterobranchia (Lankester, 1877). By the turn of the twenty-first century, enteropneusts were thought to comprise about 100 species divided among three families: Spengelidae (Willey et al., 1899), Ptychoderidae (Spengel et al., 1893), and Harrimaniidae (Spengel et al., 1902). Most of these inhabited shallow marine environments, and only three deep-sea acorn worms were then known-a spengelid (Spengel, 1893), a ptychoderid (Belichov, 1971), and a harrimaniid (Cannon et al., 2009). During the last fifteen years, however, more and more deep-sea enteropneusts have been described (Holland et al., 2005, 2009, 2012a, 2012b, 2013)(Priede et al., 2012)(Osborn et al., 2012, 2013)(Jabr et al., 2018), most of them in a new, exclusively deep-living family, Torquaratoridae. These recent discoveries have also expanded conceptions about enteropneust ecology because the torquaratorids, in contrast their shallow, infaunal relatives, crawl exposed on the deep ocean floor, although they can temporarily ascend into the water column to drift demersally, presumably to reach new epibenthic foraging sites (Smith et al., 2005) (Priede et al., 2012)(Jones et al., 2013).
Enteropneusts in general and those living in the deep sea in particular are fragile; consequently specimens collected by dredge or box core, while suitable for molecular characterization, are often too damaged for anatomical study. This problem can be largely circumvented by the use of remote operating vehicles (ROVs). With this technology, the worms can be video-recorded on the deep ocean floor, and then the very same specimens can be captured and brought to the surface sufficiently intact for detailed anatomical descriptions in addition to molecular analyses.
The present paper deals with a torquaratorid enteropneust and an acoelomorph worm living in its pharyngeal lumen. The specimens were collected from abyssal depths in the southern Gulf of California, Mexico. Both the host acorn worm and its presumed commensal acoelomorph are new species belonging to previously described genera. The discovery of an acoelomorph that lives commensally as well as at abyssal depths is doubly noteworthy because almost all the previously described species in this clade are free-living and inhabit nearshore environments (Jennings et al., 1971).
Materials and Methods
The abyssal fauna of the Gulf of California was surveyed during a MBARI expedition (from February 4 to May 10, 2012) utilizing the research vessel Western Flyer and the ROV Doc Ricketts. Video recordings made during ROV deployments were subsequently evaluated and cataloged for location, depth and species by the Video Annotation and Reference System (Schlining et al., 2006). During the expedition, scores of specimens of a previously unknown enteropneust species were video-recorded at abyssal depths. On 24 February 2012, during dive 342, two of these worms were collected after having been recorded on video. These were the holotype at 3245 m (MBARI video archive D0342-03HD) and the paratype at 3242 m (MBARI video archive D0342-02HD). After collection by suction sampler, the two acorn worms were brought to the surface. Within minutes of reaching the surface, the color in life under daylight illumination was noted. Small volumes of tissue were taken from each and preserved in M2 extraction buffer (Auto Gen, Holliston, MA) or chilled 95% ethanol and frozen for genetic analysis. In addition, living acoelomorph symbionts (evidently released through a tear in the gut wall of the holotype) were preserved as above for molecular work. Subsequently, most of the body of the acorn worms and several entire acoelomorphs were fixed in 5% formalin sea water for morphological study.
For histological processing, the fixed holotype of the acorn worm was embedded in paraplast wax and cut on a rotary microtome as serial cross sections 15-µm thick. In addition, the inner surface of a mid-dorsal fragment of the pharynx of the paratype was photographed as a whole mount to show the arrangement of the gill bars as seen from the pharyngeal lumen. Acorn worm oocytes, which shattered when sectioned in wax, and the acoelomorphs were embedded in Spurr’s resin and cut 4-µm thick with glass knives. The wax and resin sections were stained in an aqueous solution of 0.1% azure A with 0.1% sodium borate and mounted, respectively, in Permount or immersion oil. Small samples of mesoderm from the esophageal region of the paratype were prepared as whole mounts by gentle compression beneath coverslips to visualize mesodermal tubules (possibly coeloms).
Genomic DNA was extracted from each specimen by a modified semi-automated organic/phenol protocol performed by an AutoGenprep965 (AutoGen, Holliston, MA). Final elution was in 100 µl of the manufacturer’s elution buffer. A 1628-bp region of the small subunit ribosomal DNA (18S) was amplified in three overlapping fragments (of 480 bp, 606 bp, and 565 bp) obtained, respectively, with the following three primer pairs: Hemi 18S-1F, 5’-GCGAATGGCTCATTAAATCAGTTATGG-3’ with Hemi 18S-1R,
5’-GCTTTAATATACGCTATTGGAGCTGG-3’; Hemi 18S-2F,
5’-ATTGGAGGGCAAGTCTGGTGCCAG-3’ with Hemi 18S-2R,
5’-TGAGTCAAATTAAGCCGCAGGCTCC-3’; and Hemi 18S-3F,
5’-CTTAAAGGAATTGACGGAAGGGCACC-3’ with Hemi 18S-3R,
5’-TGATCAAGTTTGATCATCTTCTCG-3’. Regions of mitochondrial 16S ribosomal DNA were amplified and sequenced with 16Sar and 16Sbr primers (Palumbi et al., 1991). The polymerase chain reaction (PCR) was carried out in 20-µl volumes using a final concentration of 0.3 µM of each primer, 3 µM MgCl2 , 0.5 µM dNTPs, 0.25 mg/µml BSA, and 0.05 U/µM of Biolase DNA polymerase (BioLine, Taunton MA) with manufacturer-provided buffers. The thermal cycler was programmed for an initial 3-min denaturing period at 95oC, followed by 40 amplification cycles, each comprising a 1-min denaturing period at 95oC, a 30-sec annealing period at 55oC (for 18S) or at 45oC, 50oC or 55oC (for 16S), and a 30-sec extension period at 72oC. A final 5-min extension period at 72oC terminated the last cycle. PCR products were neutralized using ExoSAP-IT (Affymetrix, Santa Clara CA) and prepared for sequencing using BigFDye Terminator v3.1 (Applied Biosystems, Foster City CA). Cycle sequenced products were purified on Sephadex GM 50 Fine (GE Healthcare, Chicago IL) and sequenced on an ABI 3730. Sequence contigs were collated and edited using Geneious Pro 6.1.6 (Drummond et al., 2008). Molecular analysis of two individuals of the associated acoelomorphs was parallel to that described above for the enteropneusts. 18S rDNA was the target genetic marker. Thalassoanaperus abyssalis n. sp. sequences were added to the data set of (Jondelius et al., 2011) to confirm taxonomic placement with reverse taxonomy.
Phylogenetic analysis of both the enteropneust and acoelomorph sequences was effected with jModelTest (Posada et al., 2008) to select a best-fit model of nucleotide substitution. At least six replicate Bayesian runs were completed for 20-60 million generations each for individual genes and for the concatenated (but unlinked) sequences in MrBayes 2.5. Node support was expressed as posterior probabilities, and nodes with support less than 0.95 were collapsed. Concatenated analyses were also run in RA × ML and 100 bootstraps were completed for each.
Results
Acorn worm: systematic account
Phylum Hemichordata Bateson
Class Enteropneusta Gegenbaur
Family Torquaratoridae
Genus Yoda
Type species Yoda purpurata.
Yoda osbornae n. sp: Type material: Holotype: ROV Doc Rickets dive 342 (24.3667, -109.2080), 3254 m, 24 February 2012. Hermaphrodite. Major portion fixed in formalin for serial histological cross sections. Histological preparations and un-sectioned regions are deposited in the National Museum of Natural History under repository number USNM 1185901. Paratype: ROV Doc Rickets dive 342 (24.3665, -109.2084), 3242 m, 24 February 2012. Hermaphrodite. Major portion (fragmented during collection) fixed in formalin. (National Museum of Natural History repository number UNSM 1185900.
Diagnosis: Yoda osbornae n. sp. is closely related Y. purpurata as shown by phylogenetic analysis as well as morphology. Importantly, these two congeners are the only enteropneusts so far known to be hermaphrodites. The former differs from the latter in having much less prominent nuchal protuberances on the anterior dorsal ridge and markedly blunter lateral lips. This last feature means that Y. osbornae, in contrast to Y. purpurata, bears no resemblance to the Star Wars character named Yoda (a cautionary tale for taxonomists who dream up cute genus names prematurely).
Etymology: Y. osbornae (Latin): to honor the memory of the late Karen Osborn, whose untimely death has robbed deep-sea biology of one of its most promising young practitioners.
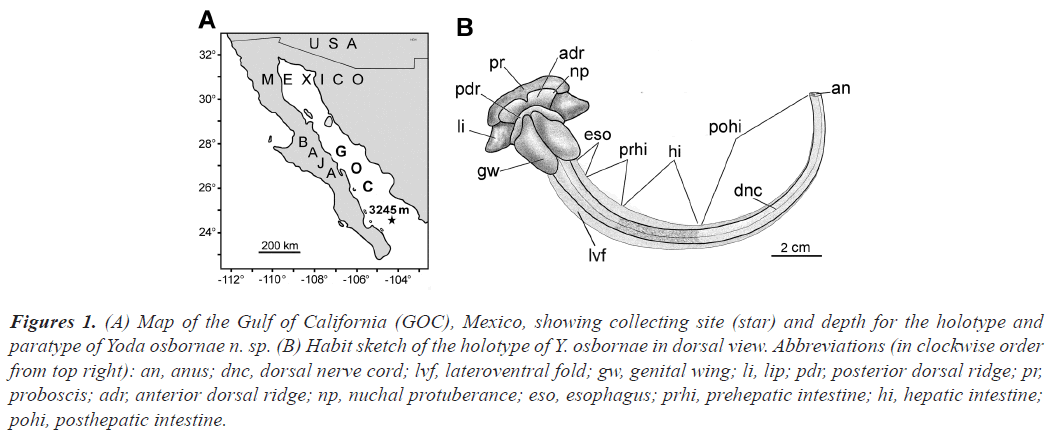
External description: Length of living holotype measured from frame grab of deep-sea video was 19.5 cm (shrinking after formalin fixation to 15.5 cm). The body color of the living animal by daylight was a dark reddish purple overall, but after formalin fixation the color changed to an orange-tinted brown. Figure 2A is a dorsal view of the formalin-fixed holotype (with levels of subsequent cross sectional figures indicated). Figure 2B is the same specimen in ventral view.
The blunt proboscis is four times wider than long. The collar with its lateral lip on either side is the widest region of the body; each lip is about a sixth as wide as the total width of the collar and tapers rapidly to a blunt point. In dorsal view, much of the collar comprises a transversely oriented anterior dorsal ridge extending on either side as an inconspicuous nuchal protuberance; just posterior to that is a less prominent posterior dorsal ridge. The ventral side of the collar consists of the posterior lip of the mouth. Posterior to the collar, most of the body length comprises the trunk regions, which are indented by a mid-ventral groove. Along the anterior tenth of the trunk, which houses the pharyngeal region of the gut, the main body axis is greatly expanded on either side as a genital wing. The two genital wings arc around dorsally until they almost meet in the dorsal midline. Posterior to the genital wings, the trunk continues through the regions of the esophagus, prehepatic intestine, hepatic intestine, and posthepatic intestine (as diagrammed in figure 1B). The trunk regions are not distinctly demarcated from one another externally (except for the slightly darker hue of the hepatic intestine) and are flanked on either side by a narrow lateroventral fold that increases the area of the ventral body wall in contact with the substrate.
Figure 1:(A) Map of the Gulf of California (GOC), Mexico, showing collecting site (star) and depth for the holotype and paratype of Yoda osbornae n. sp. (B) Habit sketch of the holotype of Y. osbornae in dorsal view. Abbreviations (in clockwise order from top right): an, anus; dnc, dorsal nerve cord; lvf, lateroventral fold; gw, genital wing; li, lip; pdr, posterior dorsal ridge; pr, proboscis; adr, anterior dorsal ridge; np, nuchal protuberance; eso, esophagus; prhi, prehepatic intestine; hi, hepatic intestine; pohi, posthepatic intestine.
Internal anatomy: The histological cross sections described below, as already mentioned, correspond to levels indicated in figure 2A. The proboscis is characterized internally by a loose meshwork of muscle and connective tissue that is locally compacted into a thin transverse lamina. No voluminous proboscis coelom, proboscis skeleton, or proboscis pores are evident. The epidermis of this body region is rich in mucous cells and underlain by the neuropile of the intraepidermal nervous system.
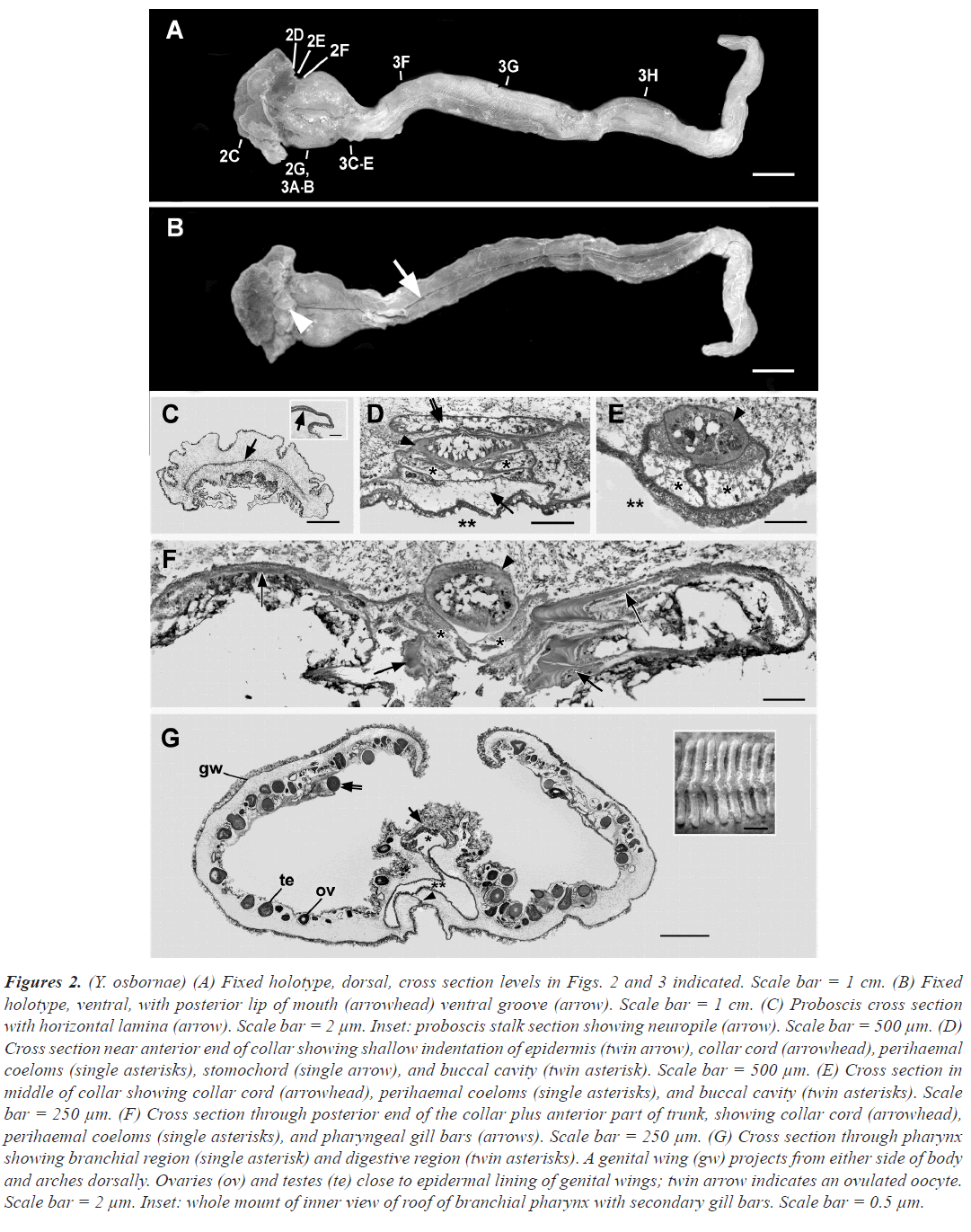
Figure 2:(Y. osbornae) (A) Fixed holotype, dorsal, cross section levels in Figs. 2 and 3 indicated. Scale bar = 1 cm. (B) Fixed holotype, ventral, with posterior lip of mouth (arrowhead) ventral groove (arrow). Scale bar = 1 cm. (C) Proboscis cross section with horizontal lamina (arrow). Scale bar = 2 µm. Inset: proboscis stalk section showing neuropile (arrow). Scale bar = 500 µm. (D) Cross section near anterior end of collar showing shallow indentation of epidermis (twin arrow), collar cord (arrowhead), perihaemal coeloms (single asterisks), stomochord (single arrow), and buccal cavity (twin asterisk). Scale bar = 500 µm. (E) Cross section in middle of collar showing collar cord (arrowhead), perihaemal coeloms (single asterisks), and buccal cavity (twin asterisks). Scale bar = 250 µm. (F) Cross section through posterior end of the collar plus anterior part of trunk, showing collar cord (arrowhead), perihaemal coeloms (single asterisks), and pharyngeal gill bars (arrows). Scale bar = 250 µm. (G) Cross section through pharynx showing branchial region (single asterisk) and digestive region (twin asterisks). A genital wing (gw) projects from either side of body and arches dorsally. Ovaries (ov) and testes (te) close to epidermal lining of genital wings; twin arrow indicates an ovulated oocyte. Scale bar = 2 µm. Inset: whole mount of inner view of roof of branchial pharynx with secondary gill bars. Scale bar = 0.5 µm.
Figure 2D is a cross section of the anterior collar region at the level of the anterior neuropore. Salient features are the collar cord (arrowhead), perihaemal coeloms (single asterisks), stomochord (arrow), and buccal cavity (twin asterisks). Near the middle of the collar, the collar cord is still underlain by perihaemal coeloms (single asterisks), but the stomochord is no longer present. Towards the posterior end of the collar, the collar cord and perihaemal coeloms are still present dorsally, while gill bars (which are trunk structures) are starting to appear ventrally. No collar pores were detected.
A cross section through the genital wing region of the trunk shows the main axis of the body in which run the dorsal and ventral haemal vessels (figure 2G arrow and arrowhead, respectively), which underlie, respectively, the inconspicuous dorsal and ventral nerve cords. In the genital wing region the pharyngeal lumen is incompletely divided into a dorsal branchial region and a ventral digestive region (single and twin asterisks, respectively). The inset in 2G shows the dorsal side of the branchial pharynx viewed from the pharyngeal lumen; the secondary gill bars are prominent.
Y. osbornae is hermaphroditic. Just beneath the epidermis on the inner surface of the genital wings, numerous ovaries (each containing a single oocyte) and testes are present (in figure 2G ov and te, respectively). Some of the largest oocytes have been extruded from the ovaries (probably prematurely ovulated due to the stress of collection). Histological details of the testes and ovaries are shown in figure 3A. Figure 3B shown an ovulated maximum-sized oocyte surrounded by a jelly layer.
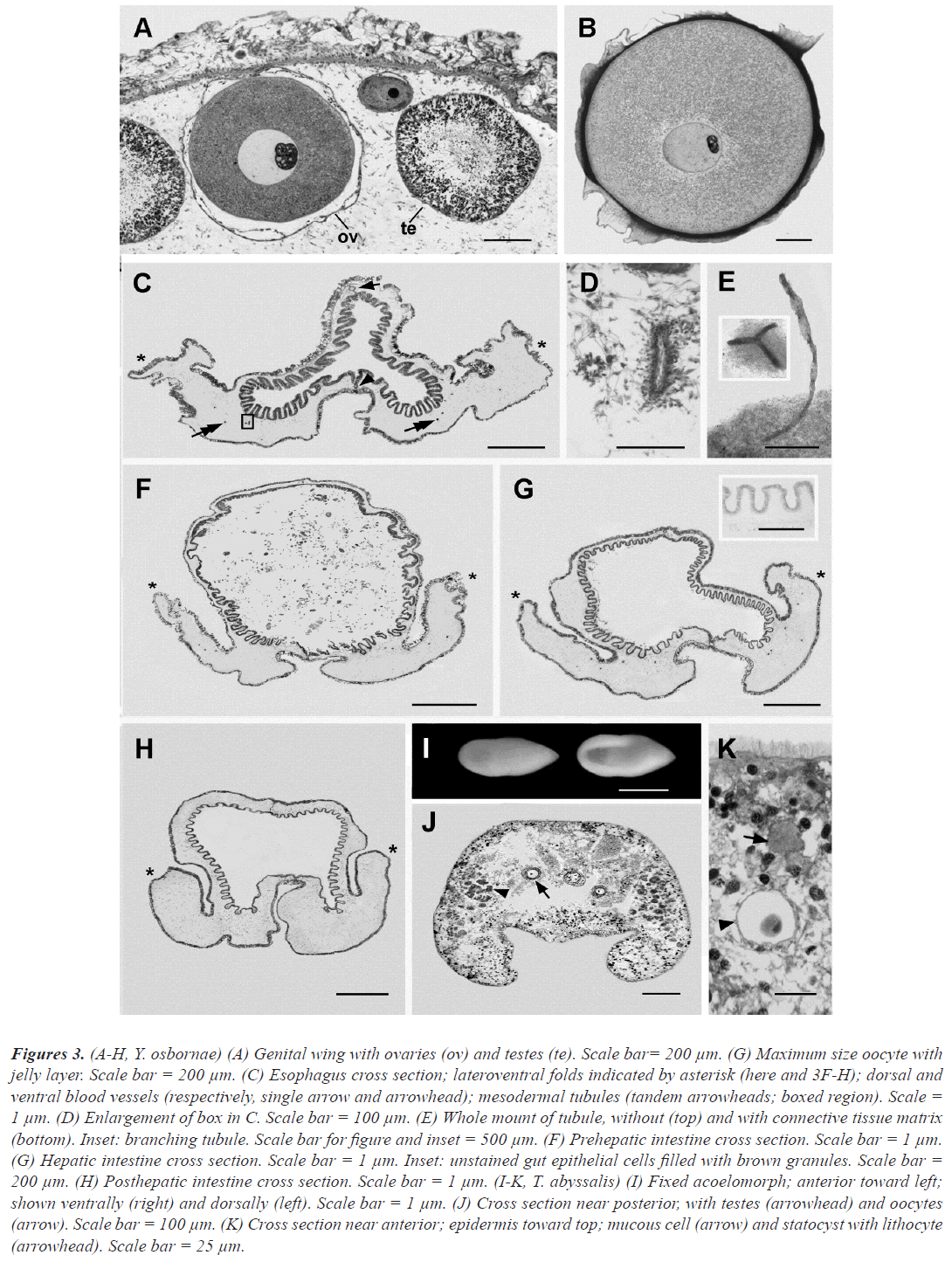
Figure 3:(A-H, Y. osbornae) (A) Genital wing with ovaries (ov) and testes (te). Scale bar= 200 µm. (G) Maximum size oocyte with jelly layer. Scale bar = 200 µm. (C) Esophagus cross section; lateroventral folds indicated by asterisk (here and 3F-H); dorsal and ventral blood vessels (respectively, single arrow and arrowhead); mesodermal tubules (tandem arrowheads; boxed region). Scale = 1 µm. (D) Enlargement of box in C. Scale bar = 100 µm. (E) Whole mount of tubule, without (top) and with connective tissue matrix (bottom). Inset: branching tubule. Scale bar for figure and inset = 500 µm. (F) Prehepatic intestine cross section. Scale bar = 1 µm. (G) Hepatic intestine cross section. Scale bar = 1 µm. Inset: unstained gut epithelial cells filled with brown granules. Scale bar = 200 µm. (H) Posthepatic intestine cross section. Scale bar = 1 µm. (I-K, T. abyssalis) (I) Fixed acoelomorph; anterior toward left; shown ventrally (right) and dorsally (left). Scale bar = 1 µm. (J) Cross section near posterior, with testes (arrowhead) and oocytes (arrow). Scale bar = 100 µm. (K) Cross section near anterior; epidermis toward top; mucous cell (arrow) and statocyst with lithocyte (arrowhead). Scale bar = 25 µm.
Posterior to the pharyngeal region, the gut continues as a relatively short esophagus characterized by a triradiate lumen (with one dorsal and two lateroventral extensions). Numerous low folds (plicae) project from luminal side of the epithelium lining the gut. The dorsal and ventral blood vessels with their associated dorsal and ventral nerve cords (already mentioned for the pharyngeal region) continue posteriorly through the esophageal region (single arrow and arrowhead, respectively) and all the rest of the trunk. The lateroventral folds (already mentioned as extending along either side of the trunk posterior to the genital wings) are indicated by asterisks in figure 3C, F-H.
The epidermis of each fold covers a voluminous core of connective tissue comprising an extracellular matrix in which is embedded a sparse network of muscles, connective tissue cells, and very inconspicuous hollow tubules (figure 3C tandem arrowheads). The tubules, which have not been mentioned previously in the acorn worm literature, are about 40 µm in outer diameter and contain an indistinct lumen about 5 µm in diameter. They branch occasionally and are blind, never merging with the gut lumen or the exterior. Such tubules are found along most of the length of the trunk, except near its posterior end. It is possible that they represent an inconspicuous remnant of the metacoel coelom of early development.
The gut region posterior to the esophagus is the prehepatic intestine. The epithelial lining is still plicate, but the lumen is approximately circular. The gut contents consist of unidentifiable fibrous and granular material in which are some scattered diatom frustules, foraminiferan tests, and sponge spicules. The prehepatic intestine merges posteriorly with the hepatic intestine, which is characterized by dorsal outpouchings that are most conspicuous in longitudinal sections. The gut epithelium surrounding the lumen of the hepatic region differs from that elsewhere in the digestive tract due to the presence of dark brown cytoplasmic granules (visible in the unstained section in the inset in figure 3G). The posthepatic intestine, which is lined by epithelial cells lacking brown cytoplasmic granules, terminates posteriorly at the anus.
Ecology: Scores of specimens of Y. osbornae were observed on the deep-sea floor near the southern end of the Gulf of California at depths from 2374 m to 3,688 m, almost always on soft substrata (K. J. Osborn, personal communication). When observed in situ, the body was usually bent into a gentle C-shape. In many, but not all, of the video-recorded worms, the features at the anterior region of the body were somewhat blurred by a thin covering of mucus lightly dusted with fine grains of substrate. The fecal trails laid down by the worms were usually sinuous or meandering, although a few spiral patterns were observed. The worms were not observed floating demersally above the bottom. Like other torquaratorids, Y. osbornae evidently deposit feeds by selectively ingesting predominantly soft material from the surface of the ocean floor. The process is apparently highly efficient because the gut lumen was almost virtually free of mineral grains (thus greatly facilitating the cutting of histological sections).
Phylogenetic analysis: An analysis based on mitochondrial 16S rDNA, nuclear 18S rDNA places Y. osbornae n. sp. close to Y. purpurata (these two species are the only hermaphroditic enteropneust species known to date). Another near relative is the so-called IFREMER enteropneust (also known as CRBHS002 or Eu72838 in other studies). It has never been described morphologically (presumably because of damage during collection), but seems very likely to belong in the genus Yoda.
Acoelomorph: systematic account
Phylum Acoelomorpha, the current nomenclature for higher intermediate clades of acoelomorphs is omitted here because it is unsettled and in need of revision (Achatz et al., 2013). For example, family Thalassoanaperidae (Dörjes et al., 1968) has been recently been subsumed into the more inclusive family Convolutida (Jondelius et al., 2011).
Genus Thalassoanaperus nom. nov. (Rivaz Hernández et al., 2018). [(Anaperus Graf 1911) was found to be preoccupied by Anaperus Troschel, 1845, Echinodermata, Holothuroidea.]
Type species: Thalassoanaperus gardeneri (Graff, 1910).
Thalassoanaperus abyssalis n. sp: Type material: ROV Doc Rickets dive 342 (24.3667N, 109.208W), 3254 m, 24 February 2012. Several acoelomorphs were found in the container with the freshly collected holotype of Y. osbornae, evidently because they had escaped from the host’s pharyngeal lumen through a collection-induced tear in the gut wall. Later histological examination of the acorn worm revealed five more of the presumed symbionts in the pharyngeal lumen of the host. Two of the freed acoelomorphs were preserved whole for molecular sequencing and another was fixed in 5% formalin-sea water. The latter was photographed intact and then embedded in Spurr’s resin and prepared as histological cross sections. The sectioned specimen was designated as the holotype (USNM 1185900a). Paratypes: the five specimens of T. abyssalis seen in serial sections through the pharynx of the holotype of the host enteropneust (deposited under the accession number of the host, UNSM 1185900).
Diagnosis: Associated (presumably as entocommensals) with deep-sea acorn worm host, thus differing from all other Thalassoanaperus species, which are free living and occur at depths less than 45 m. The strongly concave ventral surface resembles that of T. biaculeatus (Boguta et al., 1970) and T. ornatus (Beltagi et al., 2001), thus differing from the relatively flat ventral surface of all the other known congeners T. gardineri (Graff et al., 1911), T. tvaerminnensis (Luther et al., 1912), T. sulcatus, T. rubellus (Westbald et al., 1945), T. australis (Westbald et al., 1952), T. singularis (Hooge et al., 2004) as well as the closely related Neochilidia fusca (Bush et al., 1975). Among Thalassoanaperus species that are strongly concave ventrally, the white body color when alive distinguishes T. abyssalis from T. biaculeatus (orange) and from T. ornatus (brown with gold, blue, and green patches).
Etymology: Species name, abyssalis, is a Latin adjective meaning of the abyss.
External description: Preserved holotype: approximately 2 mm long by 0.8 mm wide, by 0.6 mm dorsoventrally; gently rounded anteriorly and tapering posteriorly; ventral surface strikingly concave; body opaque white; ocelli are evidently absent and no internal organs are visible through the opaque exterior.
Internal structures: The histological details of T. abyssalis fixed in 5% formalin-sea water were poorly preserved, and the position of the mouth could not be determined with certainty. The peripheral tissues (ectocytium) were denser than the internal parts (endocytium), which included considerable empty spaces that were evidently artefacts. Numerous testes were visible along the lateral regions of the body (arrowhead). The testes toward the anterior end of the body contained male germinal cells in earlier stages of spermatogenesis, whereas the more posterior testes were packed with filamentous sperm. A few oocytes with diameters up to 60 µm were located more centrally and toward the posterior end of the body. No other parts of the reproductive system could be distinguished. Near the anterior end of the body, a statocyst containing a lithocyte was visible. Elements of the nervous system were not distinguishable due to the poor fixation. Cells containing sulfated mucus (as judged by their metachromasia after azure A staining) were scattered beneath the epidermis, especially toward the anterior end of the body.
Ecology: The specimens of T. abyssalis that escaped from the gut lumen of Y. osbornae were observed alive in a dish of sea water. They glided smoothly along the bottom of the dish, traveling a few body lengths per second, evidently through the coordinated beating of the abundant epidermal cilia (Osborn, personal communication). It is very likely that the acoelomorphs eat some of the gut contents of their host, but the possibility that they also ingest gut epithelial cells of the acorn worm cannot be ruled out from the available data.
Phylogenetic analysis: Analysis based on mitochondrial 16S rDNA and nuclear 18S rDNA, placed T. abyssalis n. sp. within the relatively inclusive Clade Convolutidae and, within the latter, grouped it with five additional Thalassoanaperus species plus Neochildia fusca. This finding is in general agreement with the acoelomorph tree (Jondelius et al.,2011), who used similar methods of analysis.
Discussion
Hemichordate phylogeny
Holland et al. (2005) introduced the Torquaratoridae as a new family of enteropneusts. In response, (Rychel et al.,2008) raised objections on both phylogenetic and ecological grounds. From a study of specimens that were suitable for molecular study (but too damaged for morphological description), she concluded that torquaratorids were actually ptychoderids that had been washed out of their infaunal burrows by submersible-induced turbulence and come to rest on the sea floor. The criticism that torquaratorids are burrowing worms has been countered by extensive field observations (Anderson et al., 2011)(Osborn et al., 2012) (Priede et al., 2012), but the placement of the torquaratorids within the ptychoderids continued to be advocated by (Cannon et al.,2009). Subsequently, however, the sister group relationship between the Torquaratoridae and Ptychoderidae gained additional support from a broader sampling of taxa and sequence analysis of 18S rDNA and 16S mitochondrial rDNA (Osborn et al., 2012)(Cannon et al., 2013).
More recently, however, (Cannon et al., 2014) returned to the earlier idea that torquaratorids are nested within the ptychoderids; this conclusion was based on sequence data for two enteropneust specimens without correlated morphology (presumably due to damaged specimens). Ultimately, the controversy over whether torquaratorids are really ptychoderids should be resolved by improved methods for constructing phylogenies (Schierwater et al., 2016) and by the accumulation of reliable data for additional new species of deep-sea acorn worms.
Acoelomorphs associated with deep sea enteropneusts
An unexpected result of the present study was the discovery that an abyssal enteropneust harbored acoelomorph associates in the pharyngeal lumen. Although inquinilism between metazoan animals in the deep sea is currently poorly understood (de Buron et al., 2004), it is likely that T. abyssalis is an entocommensal. This relationship is noteworthy for two reasons. First, over 98% of all known acoelomorph species are free living, so association described here is very unusual, and, second, all of the two percent of acoelomorph species previously known to be commensal occur in relatively shallow water (Jennings et al., 1971). There has only been one previous report of an acoelomorph living in the deep sea: (Holland et al., 2005) found that the holotype of the abyssal acorn worm, Torquarator bullocki, hosted several acoelomorphs in its pharynx. The presumed commensals were not fixed well enough for a formal species description and, due to formalin-fixation, were not suitable for molecular analysis. It will be interesting to see if additional work will reveal further examples of acoelomorphs living entocommensally with abyssal acorn worms.
Acknowledgement
I am indebted to Linda A. Kuhnz and the late Karen P. Osborn for shipboard manipulations and for directing much of the molecular phylogeny. I also thank Greg Rouse for photographing the fixed specimens of Y. osbornae and T. abyssalis. The MBARI expeditions to the Gulf of California were funded by the David and Lucile Packard Foundation.
Data Availability
Inquiries about the molecular phylogeny are directed to Matthew Kweskin, Smithsonian Institution of the National Museum of Natural History, Washington, D. C. (kweskinm@ si.edu).
References
- Bateson, W. (1885). Note on the later stages in the development of Balanoglossus Kowalevskii (Agassiz), and on the affinities of the Enteropneusta. Proc. Roy. Soc. Lond. 38: 23-30.
- Willey, A. (1899). Remarks on some recent work on the Protochorda, with a condensed account of some fresh observations on the Enteropneusta. Quart. J. Mic. Sci. 42: 223-244.
- Spengel, J. W. (1893). The latest entero of the Gulf of Naples and the adjoining sea sections. Fri lander.1:37.
- Spengel, J. W. (1902). The naming of the Entero pneustengattungen. Zool. Jahrb. Abt. Syst. Geogr. Biol. Thiere. 15: 209-218.
- Belichov, D.V. Intestinal (Enteropneusta) Kuril-Kamchatka Depression (Tuscarora), Glossobalanus tuscarorae n. cn. Wop. Zool., Ser. 1971;61:3-8.
- Cannon, J. T., Rychel, A.L., Eccleston, H., Halanych, K. M., and Swalla, B. J. (2009). Molecular phylogeny of Hemichordata, with updated status of deep-sea enteropneusts. Mol. Phylog. Evol. 52: 17-24.
- Priede, I. G., Osborn, K. J., Gebruk, A. V., Jones, D., Shale, D., Rogacheva, A., and Holland, N. D. (2012). Observations on torquaratorid acorn worms (Hemichordata, Enteropneusta) from the North Atlantic with descriptions of a new genus and three new species. Invert. Biol. 131: 244-257.
- Osborn, K. J., Kuhnz, L. A., Priede, I. G., Urata, M., Gebruk, A. V., and Holland, N. D. (2012). Diversification of acorn worms (Hemichordata, Enteropneusta) revealed in the deep sea. Proc. Roy. Soc. Lond. B 279: 1646-1654.
- Osborn, K. J., Gebruk, A.V., Rogacheva, A., and Holland, N.D. (2013). An externally brooding acorn worm (Phylum Hemichordata, Class Enteropneusta, Family Torquaratoridae) from the Russian Arctic. Biol. Bull. 225: 113-123.
- Jabr, N., Archambault, P., and Cameron, C. B. (2018). Biogeography and adaptations of torquaratorid acorn worms (Hemichordata: Enteropneusta) including two new species from the Canadian Arctic. Can. J. Zool. 96:1221-1229.
- Smith, K. L., Holland, N. D., and Ruhl, H. A. (2005). Enteropneust production of spiral fecal trails on the deep-sea floor observed with time-lapse photography. Deep Sea Res. 52: 1228-1240.
- Jones, D. O. B., Alt, C. H. S., Priede, I. G., Ried, W. D. K., Wigham, B. J., Billett, D. S. M., Gebruk, A. V., Rogacheva, A., and Gooday, A. J. (2013). Deep-sea surface-dwelling enteropneusts from the Mid-Atlantic Ridge: Their ecology, distribution and mode of life. Deep Sea Res. 98: 374-387.
- de Buron, I. and Morand, S. (2004). Deep-sea hydrothermal vent parasites: why do we not find more?. Parasitol. 128: 1-6.
- Jennings, J. B. 1971. Parasitism and commensalism in the Turbellaria. Adv. Parasitol. 9: 1-32.
- Schlining, B. and Stout, N. (2006). MBARI’s video annotation and reference system. Institute of Electrical and Electronics Engineers Ocean Conference, Boston.
- Palumbi, S. R., Martin, A. P., Romano, S. L., McMillan, W. O., Stice, L., and Grabowski, G. (1991). The Simple Fool’s Guide to PCR. Dept. of Zoology, University of Hawaii, Honolulu.
- Drummond, A. J., Ashton, B., Cheung, M., Heled, J., Kearse, M., Moir, R., Stones-Havas, S., Thierer, T., and Wilson, A. (2008). Geneious. 6:1-6.
- Jondelius, U., Wallberg, A., Hooge, M., and Raikova, O. (2011). How the worm got its pharynx: phylogeny, classification and Baysian assessment of character evolution in Acoela. Syst. Biol. 60: 845-871.
- Posada, D. (2008). jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 25: 1253-1256.
- Achatz, J. G., Chiodin, M., Salvenmoser, W., Tyler, S., and Martinez, P. (2013). The Acoela: on their kind and kinships, especially with nemertodermatids and xenoturbellids (Bilateria incertae sedis). Org. Div. Evol. 13: 267-286.
- Dörjes, J. (1968). Die Acoela (Turbellaria) der deutschen Nordseeküste und ein neue System der Ordnung. Z. Zool. Syst. Evol. 6: 56-452.
- Rivaz Hernández, J. A. (2018). New replacement name for Anaperus Graff, 1911 (Acoelomorpha: Acoela: Convolutidae). Zootaxa. 418: 499-500.
- Graf, L. von. (1910). Comparison of the North American and European turbellarian fauna. Proc. Sev Int. Cong. Zool. 19-24.
- Graff, L. von. (1911). Acoela, Rhabdocoela and Alloeocoela of the eastern United States of America. Z. wiss. Zool. 99: 1-108.
- Boguta, K. K. (1970). Novaja turbelljarija Anaperus biaculeatus sp. n. (Turbellaria, Acoela) from the sublittorals of the White Sea. Zool. 49: 198-208.
- Beltagi, S. (2001). Anaperus ornatus n. sp.: a new interstitial acoelan Turbellaria from marine sediments of the Red Sea. Mar. Sci. 12: 163-174.
- Luther, A. (1912). Studies on ale tubellarians from the Gulf of Finland. Acta Soc. 36: 1-60
- Westbald, E. (1945). Studies of Scandinavian Turbellaria Acoela. Ark. Zool. 36 : 1-54.
- Westbald, E. (1952). Turbellaria (exc. Kalyptorhynchia) of the Swedish South Polar Expedition 1901-1903. Furth Zool Res Swed Antarc Exp. 4: 1-55.
- Hooge, M. D., and Smith, J. P. S. (2004). New acoels (Acoela, Acoelomorpha) from North Carolina. Zoo taxa. 442: 1-24.
- Bush, L. (1975). Biology of Neochilida fusca n. gen., n. sp. from the northeastern coast of the United States (Plathelminthes: Turbellaria). Biol. Bull. 148: 35-48.
- Rychel, A. L. (2008). Looking for the deuterostome ancestor: hemichordate phylogeny, cartilage development and regeneration. PhD Dissertation, University of Washington, Seattle.
- Anderson, T. J., Przeslawski, R., and Tran, M. (2011). Distribution, abundance and trail characteristics of acorn worms at Australian continental margins. Deep-Sea Res. 58: 970-978.
- Osborn, K. J., Kuhnz, L. A., Priede, I. G., Urata, M., Gebruk, A. V., and Holland, N. D. (2012). Diversification of acorn worms (Hemichordata, Enteropneusta) revealed in the deep sea. Proc. Roy. Soc. Lond. B 279: 1646-1654.
- Cannon, J. T., Swalla, B. J., and Halanych, K. M. (2013). Hemichordate molecular phylogeny reveals a novel cold-water clade of harrimaniid acorn worms. Biol. Bull. 225: 194-204.
- Cannon, J. T., Kokot, K. M., Waits, D. S., Weese, D. A., Swalla, B. J., Santos, S. R., and Halanych, K. M. (2014). Phylogenomic resolution of the hemichordate and echinoderm clade. Curr Biol. 24: 2827-2832.
- Schierwater, B., Holland, P. W., Miller, D. J., Stadler, P. F., Wiegmann, B. M., Wörheide, G., Wray, G. A., and DeSalle, R. (2016). Never ending analysis of a century old evolutionary debate: “unringing” the urmetazoan bell. Front. Ecol. Evol. 4: 5.
- Holland, N.D., Clague, D. A., Gordon, D. P., Gebruk, A., Pawson, D.L., and Vecchione, M. (2005). “Lophenteropneust” hypothesis refuted by collection and photos of new deep-sea hemichordates. Nature 434: 374-376.
- Holland, N. D., Jones, W. J., Ellena, J., Ruhl, H. A., and Smith, K. L. (2009). A new deep-sea species of epibenthic acorn worm (Hemichordata, Enteropneusta). Zoo systema. 31: 333-346.
- Holland, N. D., Khunz, L. A., and Osborn, K. J. (2012a). Morphology of a new deep-sea acorn worm (Class Enteropneusta, Phylum Hemichordata): a part-time demersal drifter with externalized ovaries. J. Morphol. 273: 661-671.
- Holland, N. D., Osborn, K. J., and Khunz, L. A. (2012b). A new deep-sea species of harrimaniid enteropneust (Hemichordata). Proc. Biol. Soc. Wash. 125: 228-240.
- Holland ND, Osborn KJ, Gebruk AV, Rogacheva A. (2013). Rediscovery and augmented description of the H. M. S. Challenger acorn worm (Hemichordata, Enteropneusta), Glandiceps abyssicola, in the equatorial Atlantic abyss. J. Mar. Biol. Ass. 93: 2197-2205.