Research Article - International Journal of Pure and Applied Zoology (2025) Volume 13, Issue 4
A Comparative Assessment of Ant Species Richness under Different Shade Coverages in the Coffee Agroecosystem
Remya Venugopal*, PR Swaran
Department of Zoology, Kannur University, Kerala, India
*Corresponding Author:
- Email: sunithas0122@gmail.com
Received: 16-Aug-2024, Manuscript No. IJPAZ-24-145587; Editor assigned: 19-Aug-2024, IJPAZ-24-145587 (PQ); Reviewed: 03-Sep-2024, QC No. IJPAZ-24-145587; Revised: 17-Aug-2025, Manuscript No. IJPAZ-24-145587 (R); Published: 24-Aug-2025, DOI: 10.35841/ijpaz-13.4.304
Abstract
Ants play key role in environment management due to their abundance, Diversity and functional importance. The present study examined the ant species diversity and relative abundance in coffee agro-ecosystem of Wayanad region of the Western Ghats (11°.27’00” and 11°.58’52” and the East Longitude 75°.47’50” and 76°.27’35”) under different intensity of canopies. Ants were recorded from August 2022 to September 2023 from all three sampling locations belonging to six coffee plantations i.e., site 1- Highly Shaded (HSC), site 2-Moderately Shaded (MSC) and site 3-open (OC) coffee plantation. Ants were sampled by using pitfall traps, honey baits, litter sifting, soil core extraction, and transect sampling methods. During the study period, a total of 5311 individual ants were collected representing 51 species in 26 genera, and six subfamilies. The distribution of ants in different subfamily showed a dominance of Formicinae with seventeen morpho-species (32%) followed by Myrmicinae (28%). Shannon- Weiner (H1) diversity index value of site 1 has 3.435 while site 2 has an H1 value of 3.477and site 3 has 3.197. Site 2 has a slightly higher H1 value than site 1 owing to its high species richness of 48 ant species and site 3 has lowest H1 value with 38 ant species. Site 3 has higher Simpson’s (D) diversity index value than site 2 and site 1. High species dominance in habitat would automatically mean that there would be low evenness.
Keywords
Ants, Coffee agro-ecosystem, Diversity, Diversity indices, Hymenoptera, Relative abundance, Species richness, Shade pattern.
Introduction
Invertebrates are potential tools in environmental management due to their abundance, diversity, and functional importance. They also exhibit complex relationships with plants and animals. Many have co-evolved with them. Because of their broad ecological role, ants are a key indicator group in studies of diversity and ecological function [1]. Ants are insects, which constitute over 75% of all estimated animal species on this planet [2]. Ants play an important role in the environment. Ants turn and aerate the soil, allowing water and nutrients to reach plant roots; in addition, they act as decomposers by feeding on organic waste, insects, or other dead animals. Ants can aid in the management and prevention of pests in agroforestry systems by acting as predators. Ant diversity is incredibly high, and these organisms are highly responsive to human impact. There are more than 9,000 described species of ants in world it is in nearly 300 genera, forming the entire family Formicidae, within the order Hymenoptera [3]. Bharti et al., reported that 828 species of ants in 100 genera from the different states in India.
The ant-plant interaction is the most basic and crucial symbiotic relation, yet plants have many times the biomass of all animals together. Ants have several functions in ecosystems including as herbivores, predators, decomposers, and nutrient cyclers [4]. Melliger et al., Uno et al., reported greater ant richness in urban forest. Beckers et al., reported techniques used to forage by ant species and classified as individual, tandem, group and mass recruitment, trunk trail and raiding. Yasuda and Koike underlined on the positive role of a single tree, that holds many arthropod species, not only ants. Flat and Weisser reported that ants tending aphids live much healthier life than others. Schatz et al., reported ant presence on fig tree diminished the parasitic wasp. Philpott et al., reported that ants help in controlling pests in the coffee agro-ecosystem in Mexico. The soil/litter fauna remains very poorly studied, especially in tropical ecosystems. And the information about spatial distribution patterns and the factors that influences species richness and abundance are scarcely available.
Coffee in India grows under a canopy of natural shade in ecologically sensitive regions of the Western and Eastern Ghats. This is one of the 25 biodiversity hot spots in the world. Coffee contributes significantly to sustaining the unique biodiversity of the region and is also responsible for the socioeconomic development of remote, hilly areas. Research in agro-ecosystems is important for multiple reasons. Studying the community assembly in highly heterogeneous tropical forests is difficult, where ant diversity is reported as highest. Alternatively, agro forests, or crop systems with trees, are typical systems for analysing, habitat choice, trophic interactions, spatial ecology, diversity, and various functional relationships like symbiosis, mutualism etc. Relative to tropical forests, agro forests are homogeneous. Habitat characteristics that affect ant assemblages in forests vary at small scales within agro ecosystems. Such variation is due to environmental manipulations for intensive agricultural practices. Physically separated coffee bushes in agro-forests represent separate habitats. Coffee cultivation in India faces challenging weather conditions, including fluctuating rain fall patterns and extended dry spells. To mitigate negative effects of these conditions, the practice of growing coffee under shade trees is advocated. Shade trees play a vital role in maintaining stable soil temperature and moisture levels protecting the coffee plants from extreme weather conditions like of high exposure and improving soil fertility. Although native to shady environments, modern coffee cultivators have a wide plasticity in response to varying irradiance. Such cultivators grow well without shade and even may show higher production than those of shaded trees, particularly in zones with adequate climate and soils [5]. This study was carried out, keeping in mind with the following objectives:
• To record the ant species richness in coffee agro-ecosystem of Wayanad region of Western Ghats.
• To compare the species richness under different shade pattern of coffee agroecosystem.
Materials and Methods
Study area
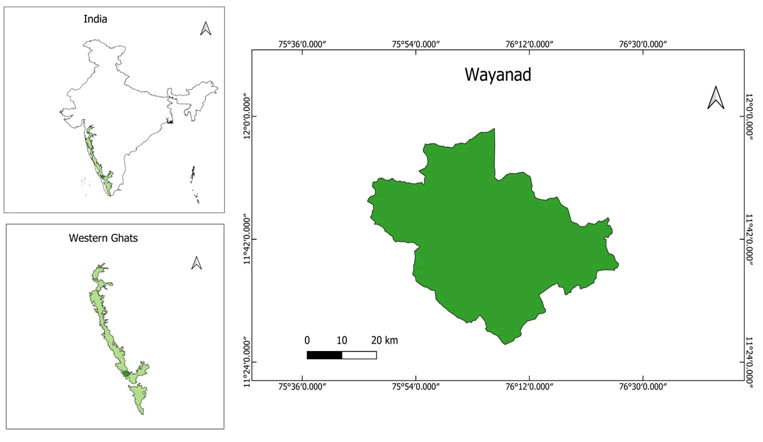
The study was carried out and the specimens were collected from the coffee agro ecosystem of Wayanad region of the Western Ghats (11°.27’00” and 11°.58’52” and the East Longitude 75°.47’50” and 76°.27’35”). Western Ghats stands second to the Eastern Himalayas as a treasure trove of biological diversity in India. Western Ghats along with its geographical extension in the wet zone of Sri Lanka is considered one of the “hottest hotspots” of biodiversity [6] and is very rich in endemic fauna and flora. Wayanad is situated at an elevation ranging from 700 mm to 2100 mm above MSL. The elevation from the sea level and the amount forest cover creates a pleasant climate in the region. During the hot weather the temperature goes to maximum 35°C and during the cold, temperature goes down to 14°C.the average rain fall is 2800 mm per year. The relative humidity is 67%. The soil is generally laterites with pH varying from 5.2-6.3. The study area was divided into three categories according to varying level of canopy cover: (a) High shaded (>70% canopy), (b) Moderately shaded (30%-60% canopy) and (c) Low shaded (<30% canopy). Ants were collected from August 2022 to September 2023 from three major sites (Site 1(HSC), Site 2(MSC), Site 3(OC)) belonging to six different coffee farms (Figures 1 and 2).
Figure 1. Map showing the study area-Wayanad region of Western Ghats.
Figure 2. Satellite image showing the sampling sites' location.
Ant sampling techniques
Ants live in different strata of the ecosystem, as their nests vary from thick leaf litter to dead wood to modified nesting structures provided by plants, tree canopies, and soil. With reference to the above, ants cannot be collected by one technique, and hence different methodologies for collection were employed. Collection techniques employed to collect the ant fauna included pitfall traps, bait technique, transect sampling with hand picking method, litter and soil extractions method, some ant species were photographed in the field itself [7].
Pitfall trap was first developed by Hertz and later enhanced by Barber. Majer suggested the combination of methods should be used for complete census of ant sampling. Wang et al., reported that sampling efficiency of pitfall traps are predominant than bait trap. Pacheco and Vasconcelos reported that subterranean traps trapped fewer species than conventional traps from the forest in Brazil. Basu employed pitfall traps in the primary forest and logged forest of Western Ghats (India). Delsinne et al., utilised ALL (Ants of the Leaf Litter) in the Ecuadorian forest and reported that moisture content in the leaf litter, might influence the abundance of ants. Fisher; Delabie et al., and Bestelmeyer endorsed the use of the Winkler method from the leaf litter of forest habitats. Groc et al., Wiezik et al., used combination of pitfall traps and Winkler method to sample forest ants. Many myrmecologists, Fisher, Ivanov et al., have favoured Winkler sacks of leaf litter. Nyamukondiwa and Addison reported that wet baits were more preferred than the dry baits, due to easy transporting condition [8].
Transect
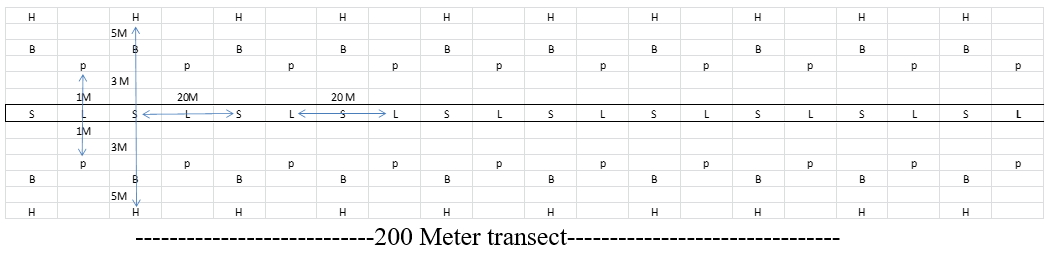
A transect of 200 meter was selected in each sampling site:
Litter sifting: Leaf litter samples were sifted in a 1 m × 1 m quadrant every 20 meters along the transect line using a litter sifter. Thus 10 samples are collected and sieved through a wire sieve with square holes of 1 cm × 1 cm and sorted and collected the ants in a jar containing 70% alcohol [9].
Soil core extract: Soil cores (20 × 20 × 15 cm) were taken at equal intervals (20 meters) along the transect. These soil cores were sifted through a hand sieve pan to collect ants.
Pitfall traps: Pitfall traps were placed in 1 meter away from the transect line on the opposite side s of the transect from where the leaf litter samples were taken. A plastic container consisting of 11 cm in diameter by 4 cm in height was placed in the hole with the lip of the trap level within the soil surface and was placed at 20 m intervals. The pitfall trap contains a small amount of soap solution to prevent the insect from escaping. The traps were kept open for 48 hours. Samples were collected and preserved in 70% ethanol and transferred to the laboratory for further identification.
Bait techniques: A small amount of sugar placed in a petridishes which was placed at in 3 meter away from the transect line on the opposite side of the line where the soil cores are collected and left open for 20 minutes to capture ants. Thus 20 petri dishes are used to collect the ants from one site. After 20 minutes the content were emptied in to polythene labelled polythene covers. This makes it easier to spot ants and capture them before they escape into the surrounding leaf litter.
Hand picking: This method is restricted to day hours (0900 hrs to 1300 hrs). Quadrates 5 m × 5m were marked along a line of transect in each 20 meters in the study area. Ants were collected from the marked quadrates immediately after baits trapping. Ants were collected using forceps, a moistened paint brush, and an inverted umbrella. The individuals are preserved in a jar containing 70% ethanol. Ants were transferred to the laboratory for further identification (Figure 3).
Figure 3. The outline of sampling points of each collection methods employed for the collection of ants in different sampling sites (S-Soil core, L-Litter sifting, P-Pitfall trap, B-Baits, H-Hand picking).
Ant morpho-species identification
For all sampling methods, ants were preserved in 70% ethanol and subsequently taken back to the laboratory for classification to species level and counting the number of species based on the morphology. Identification of ants into subfamilies, genera, species, or morpho-species was based on the keys by Bolton, the specimens were also sent to the ant research labs of Punjab University, Pattiala, and IISER, Bangalure, for authentication.
Results
Ant diversity analysis
The α-diversity reflected in this study is the values of the species richness and relative abundance for three major collection sites (site 1, site 2 and site 3). Thus, site 1 is considered in this study as a local site with high shade density site 2 is a local site with moderately shade density and site 3 is open/exposed coffee plantation.
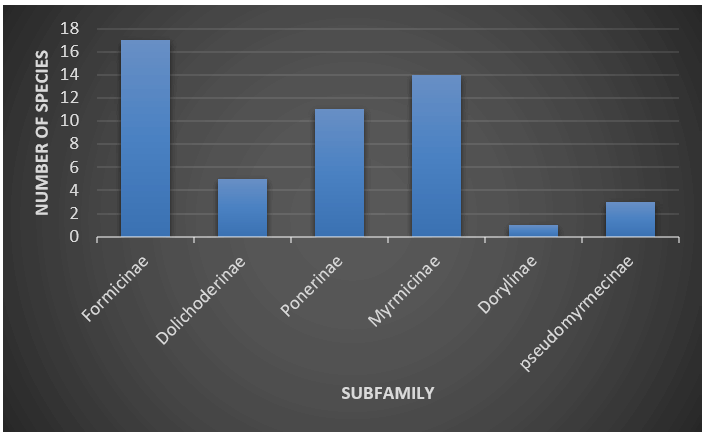
During the study period, a total of 5311 individuals were collected, representing 51 species in 26 genera and six subfamilies. The distribution of species in the different subfamilies showed a dominance of Formicinae with seventeen morphospecies (33%) followed by Myrmicinae (27%), Ponerinae (22%), Dolichoderinae (10%), Pseudomyrmecinae (6%) and Dorylinae (2%). The subfamily Myrmicinae exhibits dominance in genus richness, with 9 genera, followed by Formicinae (7 genera), Ponerinae (6 genera) Dolichoderinae (2 genera) and single genus represented by the family Pseudomyrmicinae and Dorylinae (Figures 4 and 5).
Figure 4. Distribution of ant species in coffee agro-ecosystem.
Figure 5. Graphical representation of ant species richness in sub families in coffee agro-ecosystem.
The most diverse ant genus is Camponotus, with 7 named species, followed by Polyrhachis (5 species), the genus Leptogenys, Tetraponera, Technonomyrmex, Crematogaster, represented by 3 species each, and Tapinoma, Brachyponera, Pheidole, Diacamma, Odontomachus, and Tetramorium, represented by 2 species. Only one species each is recorded from the genus Myrmicaria, Oecophylla, Anoplolepis, Paratrechina, Lepisiota, Nylanderia, Pseudoponera, Carebra, Solonopsis, Meranoplus, Cataulacusk, and Dorylus. Two monotypic exotic genera, Anoplolepis and Paratrechina, were recorded in this study. Most of the ant species found in this study have been reported as generalized foragers and predators (Table 1).
| Species | Frequency site 1 | Frequency site 2 | Frequency site 3 | Total | Rank abundance |
| Oecophylla smaragdina | 136 | 137 | 114 | 387 | 5 |
| Camponotus parius | 13 | 33 | 24 | 70 | 26 |
| Camponotus irritans | 42 | 53 | 36 | 131 | 11 |
| Anoplolepis racillipe | 66 | 99 | 80 | 245 | 6 |
| Paratrechina longicornis | 12 | 22 | 18 | 52 | 31 |
| Polyrhachis rastellata | 13 | 4 | 7 | 24 | 37 |
| Polyrhachis wroughtonii | 5 | 2 | 0 | 7 | 41 |
| Camponotus singularis | 2 | 4 | 6 | 12 | 40 |
| Camponotus radiatus | 10 | 4 | 8 | 22 | 38 |
| Camponotus sericeus | 9 | 6 | 7 | 22 | 38 |
| Camponotus angusticollis | 0 | 1 | 0 | 1 | 44 |
| Polyrhachis exercita rastrata | 23 | 8 | 14 | 45 | 32 |
| Polyrhachis tibialis | 28 | 34 | 9 | 71 | 25 |
| Polyrhachis puctillata | 0 | 0 | 2 | 2 | 43 |
| Lepisiota opaca pulchella | 10 | 16 | 0 | 26 | 36 |
| Nylanderia yerburyi | 12 | 26 | 0 | 38 | 33 |
| Camponotus compressus | 22 | 28 | 17 | 67 | 27 |
| Technomyrmex albipes | 123 | 163 | 129 | 415 | 2 |
| Technomyrmex bicolor | 20 | 3 | 0 | 23 | 38 |
| Technomyrmex elatior | 23 | 32 | 0 | 55 | 29 |
| Tapinoma indicum | 71 | 77 | 44 | 192 | 7 |
| Tapinoma melanocephalum | 143 | 172 | 143 | 458 | 1 |
| Brachyponera luteipes | 23 | 34 | 8 | 65 | 28 |
| Leptogenys diminuta | 34 | 48 | 25 | 107 | 17 |
| Pseudoponera | 0 | 0 | 2 | 2 | 43 |
| Leptogenys processionalis | 37 | 47 | 33 | 117 | 14 |
| Diacamma rugossum | 36 | 42 | 25 | 103 | 18 |
| Leptogenys birmana | 11 | 6 | 0 | 17 | 39 |
| Diacamma ceylonese | 27 | 46 | 21 | 94 | 19 |
| Odontomachus simillimus | 40 | 52 | 38 | 130 | 12 |
| Odontomachus haematodes | 61 | 66 | 40 | 167 | 9 |
| Brachyponera jerdonii | 32 | 48 | 41 | 121 | 13 |
| Harpegnathos saltator | 0 | 17 | 12 | 29 | 35 |
| Crematogaster rogenhoferi | 132 | 148 | 120 | 400 | 4 |
| Crematogaster walshi | 0 | 61 | 48 | 109 | 16 |
| Myrmicaria brunnea | 130 | 159 | 123 | 412 | 3 |
| Pheidole indica | 33 | 51 | 28 | 112 | 15 |
| Pheidole sps 2 | 16 | 16 | 0 | 32 | 34 |
| Monomorium wrouhtoni | 24 | 32 | 16 | 72 | 24 |
| Monomorium indicum | 17 | 36 | 0 | 53 | 30 |
| Crematogaster aberrans | 34 | 38 | 21 | 93 | 20 |
| Carebra diversa | 58 | 76 | 35 | 169 | 8 |
| Tetramorium coonoorense | 29 | 40 | 13 | 82 | 22 |
| Tetramorium walshi | 0 | 4 | 0 | 4 | 42 |
| Solonopsis germinate | 16 | 26 | 23 | 65 | 28 |
| Meranoplus bicolour | 70 | 42 | 20 | 132 | 10 |
| Cataulacus taprobanae | 16 | 16 | 0 | 32 | 34 |
| Dorylus orientalis | 4 | 0 | 0 | 4 | 42 |
| Tetraponera allaboras | 27 | 38 | 0 | 65 | 28 |
| Tetraponera nigra | 35 | 36 | 12 | 83 | 21 |
| Tetraponera rufonigra | 20 | 39 | 16 | 75 | 23 |
| Total | 1745 | 2188 | 1378 | 5311 |
Table 1. The corresponding frequency of ant’s species collected from the sites between August 2022 to September 2023, Wayanad.
The highest number of species and individuals was reported in moderately shaded coffee plantations, followed by shaded and the least in openly shaded coffee plantations. The moderately shaded coffee plantation has optimum temperature, humidity, and tree connectivity for ant activities compared to the thick and open shade. Filter shade and litter increase the availability of nesting sites and foraging areas of ants.
The highest number of species and individuals was reported in moderately shaded coffee plantations, followed by shaded and the least in openly shaded coffee plantations. The moderately shaded coffee plantation has optimum temperature, humidity, and tree connectivity for ant activities compared to the thick and open shade. Filter shade and litter increase the availability of nesting sites and foraging areas of ants.
Shannon-Weiner (H1 ) diversity index
Table 2 shows the Shannon-Weiner (H1 ) species diversity index for the three sites. Site 1 has an H1 value of 3.435 while site 2 has an H1 value of 3.477 and site 3 has 3.197. Site 2 has a slightly higher H1 value than site 1 owing to its high species richness of 48 ant species and site 3 has lowest H1 value with 38 ant species. With these species richness results, the evenness of ant species distribution is better in site 2. ShannonWeiner index increases as both the richness and the evenness of the community increase.
|
|
HSC |
MSC |
OC |
|
Taxa_S |
45 |
48 |
38 |
|
Individuals |
1745 |
2188 |
1378 |
|
Dominance_D |
0.0427 |
0.0397 |
0.0553 |
|
Simpson_1-D |
0.9573 |
0.9603 |
0.9446 |
|
Shannon_H |
3.435 |
3.477 |
3.197 |
|
Evenness_e^H/S |
0.6895 |
0.6744 |
0.6434 |
Table 2. Showing the diversity indices of ants under three shade coverage.
Simpson’s (D) diversity index
Simpson’s (D) diversity index is shown in Table 3. Site 1 has a D value of 0.957 while site 2 has a D value of 0.960 and sit 3 has 0.944. Just like H1 , D also takes into consideration the two components of diversity, species richness, and relative abundance. High species dominance in habitat would automatically mean that there would be low evenness. Site 3 has higher D value than site 2 and site 1 since it has a higher percentage of composition of organisms relative to the total number of organisms in the area.
| Sub family | Genus | Name of the species | HSC | MSC | LSC | Status |
| Formicina | Oecophylla | Oecophylla smaragdina | + | + | + | Native |
| Camponotus | Camponotus parius | + | + | + | Native | |
| Camponotus irritans | + | + | + | Native | ||
| Camponotus singularis | + | + | + | Native | ||
| Camponotus radiates | + | + | + | Native | ||
| Camponotus sericeus | + | + | + | Native | ||
| Camponotus angusticollis | _ | + | _ | Native | ||
| Camponotus compresses | + | + | + | Native | ||
| Anoplolepis | Anoplolepis gracilipes | + | + | + | Invasive | |
| Paratrechina | Paratrechina longicornis | + | + | + | Invasive | |
| Polyrhachis | Polyrhachis rastellata | + | + | _ | Native | |
| Polyrhachis exercita | + | + | + | Native | ||
| Polyrhachis wroughtonii | + | + | + | Native | ||
| Polyrhachis tibialis | + | + | + | Native | ||
| Polyrhachis punctillata | _ | _ | + | Native | ||
| Lepisiota | Lepisiota opaca pulchella | + | + | _ | Native | |
| Nylanderia | Nylanderia yerburyi | + | + | _ | Native | |
| Dolichoderinae | Technomyrmex | Technomyrmex albipes | + | + | + | Native |
| Technomyrmex bicolor | + | + | _ | Native | ||
| Tapinoma | Technomyrmex elatior | + | + | _ | Native | |
| Tapinoma indicum | + | + | + | Native | ||
| Tapinoma melanocephalum | + | + | + | Native | ||
| Ponerinae | Brachyponera | Brachyponera luteipes | + | + | + | Native |
| Brachyponera jerdonii | + | + | + | Native | ||
| Leptogenys | Leptogenys diminuta | + | + | + | Native | |
| Leptogenys birmana | + | + | _ | Native | ||
| Leptogenys processionalis | + | + | + | Native | ||
| Pseudoponera | Pseudoponera | _ | _ | + | Native | |
| Diacamma | Diacamma ceylonense | + | + | + | Native | |
| Diacamma rugosum | + | + | + | Native | ||
| Odontomachus | Odontomachus simillimus | + | + | + | Native | |
| Odontomachus haematodus | + | + | + | Native | ||
| Harpegnathos | Harpegnathos saltator | _ | + | + | Native | |
| Myrmicina | Crematogaster | Crematogaster rogenhoferi | + | + | + | Native |
| Crematogaster aberrans | + | + | + | Native | ||
| Crematogaster walshi | _ | + | + | Native | ||
| Myrmicaria | Myrmicaria brunnea | + | + | + | Native | |
| Pheidole | Pheidole indica | + | + | + | Native | |
| Pheidole sps 2 | + | + | _ | Native | ||
| Monomorium | Monomorium wroughtoni | + | + | + | Native | |
| Monomorium indicum | + | + | _ | Native | ||
| Carebra | Carebara diversa | + | + | + | Native | |
| Tetramorium | Tetramorium coonoorense | + | + | + | Native | |
| Tetramorium walshi | _ | + | _ | Native | ||
| Solonopsis | Solenopsis geminata | + | + | + | Native | |
| Meranoplus | Meranoplus bicolor | + | + | + | Native | |
| Cataulacus | Cataulacus taprobanae | + | + | _ | Native | |
| Dorylinae | Dorylus | Dorylus orientalis | + | _ | _ | Native |
| Pseudomyrmecinae | Tetraponera | Tetraponera | + | + | _ | Native |
| Tetraponera nigra | + | + | + | Native | ||
| Tetraponera rufonigra | + | + | + | Native | ||
| Total | 45 | 48 | 38 | |||
| Note: HSC: High Shaded Coffee; MSC: Moderately Shaded Coffee; OC-Limited shaded Coffee. | ||||||
Table 3. List of the ant species collected from the coffee agro-ecosystem of the Wayanad Region of Western Ghats.
Discussion
Ants play key role in assessing ecological response to disturbance. The present study on diversity of ants in coffee agro-ecosystem in Wayanad region of Western Ghats, revealed that there are fifty-one species of ants belonging to twenty-six genera and six subfamilies. The diversity analysis exposes that, the species diversity was comparatively high in moderately shaded coffee plantations than other sites. Subfamily Forrmicinae has been found to be most diverse with 17 species grouped in 7 genera. This subfamily represents a maximum catch with 27% generic richness and 33% species richness among the recorded six subfamilies.
The other major subfamilies include Myrmicinae (generic richness 34%, species richness 27%) and Ponerinae (generic richness 23%, species richness 22%). The subfamily Dolichoderinae exhibit generic richness of 8% and species richness of 10% and the sub families Pseudomyrmicinae (generic richness of 4% and species richness of 6%) and Dorylinae (generic richness of 4% and species richness of 2%) are comparatively less diverse. In sub family Formicinae, genera Camponotus and polyrhachis are most speciose with 7 and 5 species respectively. Ants under this genus can deed with vide range of niches and many of them are generalists and arboreal. Niche and food requirements are one of the primary limiting factors of an ant population. In the case of subfamily Myrmicinae the genera crematogaster, myrmicaria and pheidole are dominant generalists and the genus Leptogenys and Diacamma are most dominant genera belonging to subfamily Ponerinae. Most of these ants are scavangers. Species, Tapinoma melanocephalum and Technomyrmex albipes belonging to sub family Dolichoderinae are the two dominant species in coffee agro ecosystem under the various ranges of shades.
Tapinoma melanocephalum is most abundant ant species in all types of coffee agr-ecosystems this observation is in accordance with ealier report of this taxon as the common group in the region irrespective of the vegetation types in Wayanad forests. Andersen, King et al., and Majer and Nichols found that ant communities in damaged ecosystems have lower species diversity and greater numbers of Dolichoderines. Sabu et al. conducted studies on the diversity of forest habitatinhabiting ants along elevations in the Wayanad Region of the Western Ghats, and twenty-nine ant species belonging to 18 genera under 6 subfamilies were reported and subfamily Formicinae was the highly specious in evergreen forests. Olson reported that the richness and diversity of leaf litter ant species have been heavily influenced by changes in the vegetation type. Bharti and Sharma carried out a detailed study on the diversity and abundance of ants along an elevation gradient in the Jammu-Kashmir Himalaya. Bharti reported 46% of the endemism of ants in the Himalayan region. Savitha et al. observed the response of ants to disturbance gradients in and around Bengaluru, India, and estimated that ant species richness and abundance were higher in the undisturbed site.
Conclusion
Ants can be effectively used as an ecosystem indicator. Studies revealed that they immediately respond to any disturbance or alteration in their environments. The distribution of ants in different subfamilies showed a dominance of Formicinae, followed by Myrmicinae and Ponerinae. The most diverse ant genus is Camponotus, with seven species, and Polyrhachis, with five species. Moderately shaded coffee plantations exhibit the highest species richness compared to shaded and open conditions this may due to tree connectivity and optimum temperature which provide ideal sites for nesting and foraging. Tapinoma melanocephalum is the dominant species in all three shade patterns. Technomyrmex albipes, Myrmicaria brunnea, Crematogaster rogenhoferi, Oecophylla smaragdina and Anoplolepis racillipe are the major ant species present in all types of ecosystems under study. Camponotus is the most specious genus with seven morpho species followed by polyhachis with five species. The present study will provide valuable information on ant species availability in the coffee agro-ecosystem. This study provides a significant contribution in the fields of ecology and entomology.
References
- Agosti D, Majer JD, Alonso LE, et al. Standard methods for measuring and monitoring biodiversity. Smithsonian Institution, Washington DC. 2000;9:204-6.
- Akbar SA. Taxonomic Studies of Ants (Formicidae: Hymenoptera) from Western Ghats of India (Doctoral dissertation, Ph. D thesis, Punjabi University, Patiala, Department of Zoology and Environmental Sciences). 2014.
- Akbar SA, Bharti H. A new species of the ant genus Carebara Westwood (Hymenoptera: Formicidae) from India. J Entomol Res Soc. 2017;19(3):35-43.
- Andersen AN. Using ants as bioindicators: multiscale issues in ant community ecology. Conserv Ecol. 1997;1(1):17.
- Ellison AM, Record S, Arguello A, et al. Rapid inventory of the ant assemblage in a temperate hardwood forest: species composition and assessment of sampling methods. Environ Entomol. 2007;36(4):766-75.
[Crossref] [Google Scholar] [PubMed]
- Delsinne T, Roisin Y, Herbauts J, et al. Ant diversity along a wide rainfall gradient in the Paraguayan dry Chaco. J Arid Environ. 2010;74(10):1149-55.
- Flatt T, Weisser WW. The effects of mutualistic ants on aphid life history traits. Ecology. 2000;81(12):3522-9.
- Fisher BL. Ant diversity patterns along an elevational gradient in the Réserve Spéciale d'Anjanaharibe-Sud and on the western Masoala Peninsula, Madagascar. Fieldiana Zool. 1998;90:39-67.
- Fisher BL. Improving inventory efficiency: a case study of leaf?litter ant diversity in Madagascar. Ecol Appl. 1999;9(2):714-31.