Research Article - Journal of Biotechnology and Phytochemistry (2017) Volume 1, Issue 1
Screening of Punica Granatum seeds for antibacterial and antioxidant activity with various extracts.
Naeem Hasan Khan*, Adriana Lee Tze Ying, Candy Goo Zhi Tian, Ooi Wei Yi, Shantini VijayabalanDepartment of Pharmacy, AIMST University, Malaysia
- *Corresponding Author:
- Naeem Hasan Khan
Department of Pharmacy
AIMST University
Malaysia
Tel: 0060 4 4298000
Mobile: +006016 9372470
E-mail: naeemhshirazi@hotmail.com
Accepted date: November 30, 2017
Citation: Khan NH, Ying ALT, Tian CGZ, et al. Screening of Punica Granatum seeds for antibacterial and antioxidant activity with various extracts. J Gastroenterol Dig Dis. 2017;1(1):1-7.
DOI: 10.35841/biotechnology-phytochemistry.1.1.1-7
Visit for more related articles at Journal of Biotechnology and PhytochemistryAbstract
The name pomegranate derives from Latin p?mum "apple" and gr?n?tum "seeded" [1]. Pomegranate is native to Iran and northeast Turkey and has been cultivated throughout the Middle East, Southern Asia and Mediterranean region for several millennia. It is also extensively grown in South China and in South East Asia. Kandahar in Afghanistan is famous for its high-quality pomegranates [2]. Pomegranate seeds are used as a spice known as anardana’(from Persian: anar + dana, pomegranate + seed), most notably in Indian and Pakistani cuisine but also as a substitute for pomegranate syrup in Persian cuisine [3]. Dried whole seeds can often be obtained in ethnic Indian subcontinent markets. Seeds of the wild pomegranate variety known as‘daru’ from the Himalayas are regarded as quality sources for this spice [4-5]. Dried seeds can be used in several culinary applications [6]. One serving (87 grams) of pomegranate seeds contains the following nutrients as described in Tables 1-4 [7-10]. The calorific value of the pomegranate seed is 83 calories per 100 grams. Pomegranate seeds are a rich source of dietary fiber (20% DV) which is entirely contained in the edible seeds [11]. Punicic acid, also known as pomegranate seed oil, is the main fatty acid which is a type of conjugated linoleic acid with potent biological effects. Pomegranates contain punicalagins and punicic acid, unique substances that are responsible for most of their health benefits [11]. A 100- g serving of pomegranate seeds provides 12% of the Daily Value (DV) for vitamin C, 16% DV for vitamin K and 10% DV for folate. Pomegranate seeds are a rich source of dietary fiber (20% DV) which is entirely contained in the edible seeds
Keywords
Punica granatum seeds, maceration, soxhlet, antibacterial, antioxidant
Introduction
The name pomegranate derives from Latin pōmum "apple" and grānātum "seeded" [1]. Pomegranate is native to Iran and northeast Turkey and has been cultivated throughout the Middle East, Southern Asia and Mediterranean region for several millennia. It is also extensively grown in South China and in South East Asia. Kandahar in Afghanistan is famous for its high-quality pomegranates [2]. Pomegranate seeds are used as a spice known as anardana’(from Persian: anar + dana, pomegranate + seed), most notably in Indian and Pakistani cuisine but also as a substitute for pomegranate syrup in Persian cuisine [3]. Dried whole seeds can often be obtained in ethnic Indian subcontinent markets. Seeds of the wild pomegranate variety known as‘daru’ from the Himalayas are regarded as quality sources for this spice [4,5]. Dried seeds can be used in several culinary applications [6]. One serving (87 grams) of pomegranate seeds contains the following nutrients as described in Tables 1-4 [7-10]. The calorific value of the pomegranate seed is 83 calories per 100 grams. Pomegranate seeds are a rich source of dietary fiber (20% DV) which is entirely contained in the edible seeds [11]. Punicic acid, also known as pomegranate seed oil, is the main fatty acid which is a type of conjugated linoleic acid with potent biological effects. Pomegranates contain punicalagins and punicic acid, unique substances that are responsible for most of their health benefits [11]. A 100- g serving of pomegranate seeds provides 12% of the Daily Value (DV) for vitamin C, 16% DV for vitamin K and 10% DV for folate. Pomegranate seeds are a rich source of dietary fiber (20% DV) which is entirely contained in the edible seeds. People who choose to discard the seeds forfeit nutritional benefits conveyed by the seed fiber and micronutrients [12]. The most abundant phytochemicals in pomegranate juice are polyphenols, including the hydrolyzable tannins called ellagitannins formed when ellagic acid and/or gallic acid binds with a carbohydrate to form pomegranate ellagitannins, also known as punicalagins [13,14]. The red color of juice can be attributed to anthocyanins, such as delphinidin, cyanidin, and pelargonidin glycosides [15]. Generally, an increase in juice pigmentation occurs during fruit ripening [16]. The phenolic content of pomegranate juice is adversely affected by processing and pasteurization techniques [17]. Phytochemicals like triterpenoids, steroid, glycosides, saponins, alkaloids, tannins, carbohydrates and vitamin C were found present in the seed extracts. These phytochemicals may contribute to the health benefits of pomegranate seeds as in cardiac problems, stomach disorders, osteoarthritis and anaemia etc [11,12]. It has been stated that pomegranate has potential antibacterial [13-20] Antioxidant properties as well [21-23].
| Energy | 72 calories |
| Carbohydrates | 16.3 grams |
| Protein | 1.5 grams |
| Fat | 1 gram |
| Fibre | 3.5 grams |
| Sugar | 11.9 grams |
| Vitamin K | 14.3 micrograms (17.9%DV)* |
| Vitamin C | 8.9 milligrams (14.8%DV)* |
| Foliate | 33 micrograms (8.3%DV)* |
| Potassium | 205 milligrams (5.9%DV)* |
| Vitamin B6 | 0.07 milligram (3.5%DV)* |
| Phosphorus | 31 milligrams (3.1%DV)* |
Table 1: Nutritional value of Pomegranate seeds.
| Components | Values (%) |
| Percentage moisture | 8.6 |
| Total lipids | 27.2 |
| Crude protein | 13.2 |
| Crude fibre | 35.3 |
| Pectin | 6 |
| Total sugars | 4.2 |
| Ash | 2 |
Table 2: Proximate composition of Pomegranate seeds.
| Minerals | Values (ppm) |
| Iron | 1.3 |
| Sodium | 6 |
| Magnesium | 12.4 |
| Potassium | 45.2 |
| Zinc | 1 |
| Copper | 1.2 |
Table 3: Trace minerals present in Pomegranate seeds.
| Fatty Acids | Values (%) |
| Caproic acid | 2.16 |
| Punicic acid | 65.3 |
| Capric acid | 0.95 |
| Lauric acid | 6.62 |
| Myristic acid | 7.56 |
| Myristoleic acid | 0.41 |
| Palmitic acid | 4.8 |
| Palmitoleic acid | 0.47 |
| Stearic acid | 2.3 |
| Linoleic acid | 6.6 |
| Oleic Acid | 5.13 |
| Bacillus subtilis. | ATCC 11774 |
| Enterococcus faecalis. | ATCC 29212 |
| Staphylococcus aureus. | ATCC 29213 |
Table 4: Fatty acids found in pomegranate seeds.
Contraindications: Due to the potential for anaphylaxis, it is suggested that pomegranate is avoided in those who are allergic to pomegranate. There is also insufficient reliable information on safety of pomegranate extracts in pregnancy and lactation and potential genotoxicity of one whole fruit extract has been reported in an animal study [11,12].
Interactions : A potential for interaction with herbs, supplements and drugs that reduce blood pressure has been suggested due to pomegranate’s possible antihypertensive effects. Pomegranate juice is also reported to have ace inhibitorlike effects suggesting caution in those being treated with this class of drugs. Human studies show that pomegranate juice has no effect on the bioavailability or pharmacokinetics of representative cytochrome p450 2c9 (cyp2c9) and cytochrome p450 3a4 (cyp3a4) substrates. Several individual cases of potential interaction with warfarin have been reported [13,14].
Aims and Objectives
The literary evidences over therapeutic agent’s research over development of antioxidants and antibiotic activity from medicinal plants inspired the investigators to aim over screening of punica granatum seeds for antibacterial and antioxidant activity with various extracts. To accomplish the stated aims, the following objectives were designed such as:
•Authentication of plant material.
•Evaluation of antimicrobial potential and antioxidant activity of various extracts of punica granatum seeds.
Materials and Methods
Collection and preparation of research material: 2 kg of pomegranate seeds (semi dried) were purchased from Little India market in Ipoh, Perak DR Malaysia. According to the shop owner, the seeds were imported from India. The seeds were stored in a refrigerator to preserve the freshness of the seeds. The seeds were thoroughly washed manually with water, at least for three times until they were free from the foreign substances including aril. Then the seeds were rinsed with distilled water for three times. The seeds were spread thoroughly on the large filter paper and dried under shade. The dried seeds were blended by using stationary jar electric blender. The seeds were blended till fine to increase the surface area for extracting solvents.
Requirement for extractions and phytochemical screening Chemicals: 98% Sulfuric Acid (R&M Chemicals, Essex, United Kingdom), Dragendorff’s reagent (R&M Chemicals, Essex, United Kingdom), Distilled water was freshly glass distilled (Genristo, Nottingham UK) in the laboratory, Chloroform (QReC), 37.0% Hydrochloric Acid (HmbG Chemicals), Ammonia (HmbG Chemicals), sodium hydroxide (R&M Chemicals, Essex, United Kingdom), Fehling’s solution A (R&M Chemicals, Essex, United Kingdom), Fehling’s solution B (R&M Chemicals, Essex, United Kingdom), Ferric chloride (R&M Chemicals, Essex, United Kingdom), Molisch’s reagent (R&M Chemicals, Essex, United Kingdom), Millon’s reagent (QReC), Sudan’s reagent (PC Laboratory Reagent), 99.9% Ethanol (HmbG Chemicals), 99% n-Hexane (R&M Marketing, Essex, UK) Molecular weight: 86.16g/mol, 98.0% Sulfuric Acid (R&M Chemicals, Essex, United Kingdom).
Equipment: Rotary evaporator, RE 300, Yamato, Digital analytical weighing balance, Mettler Toledo, Soxhlet apparatus, Duran Group, Hot plate, Erla, Autoclave machine, Tomy. E S 315,Shaking incubator, Daiki science ltd., Ultraviolet spectrometer, Dragendorff’s reagent (R&M Chemicals, Essex, United Kingdom), Chloroform (QReC), 37.0% Hydrochloric Acid (HmbG Chemicals), Ammonia (HmbG Chemicals).
Extraction of Punica granatum seeds of Maceration with absolute ethanol: 81 grams of crushed P. granatum seeds were dried, crushed and transferred to the round bottom flask and extracted with 400 ml of absolute ethanol was measured and added into the round bottom flask. The material was completely soaked in absolute ethanol. The sample was shaken well and was left for 72 hours.
Maceration with hexane: 55 grams of crushed Punica granatum seeds was weighed and transferred to 1L round bottom flask and extracted with 300 ml of hexane was measured and added to the flask. The sample was shaken well and was left for 72 hours.
Soxhlet extraction: 84 grams of dried and crushed Punica granatum seeds were placed inside the thimble and extracted with 500 ml of absolute alcohol for about 48 hours at 55°C. The following short names were assigned for different extracts;
Es - Ethanol Soxhlet Extract
Em - Ethanol Maceration Extract
Hx - Hexane Maceration Extract
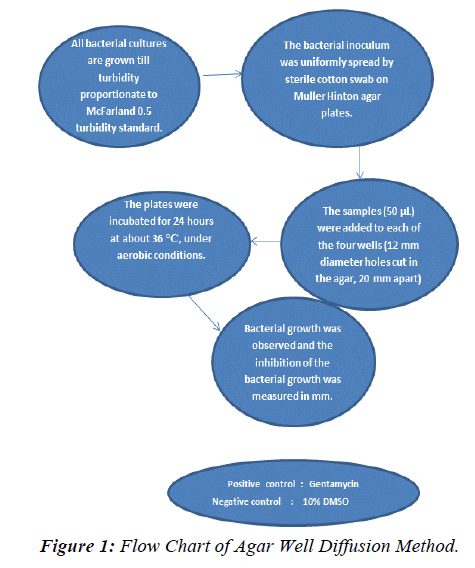
Determination of Antimicrobial Activity [19,20]
The antimicrobial activity of the ethanol and hexane extracts of Punica granatum was determined by using the well diffusion method. Antimicrobial activity was determined by measuring the zone of inhibition and the results obtained from standard antibiotic and extract were compared. Turbidity of bacterial suspension was 50 μl (antibiotic samples 1mg/ml), 0.5 McFarland standard. The volume of material used to fill the holes was 50 μl. Turbidity of bacterial suspension was 0.5 McFaland turbidity standard.
Chemicals
Mueller Hinton II Agar (Becton Dickinson, France, SA), LB Broth (HIMEDIA). Dimethyl sulfoxide, DMSO ( Riendemann Chmidt Chemical), 70% Ethanol (HmbG Chemicals), Extracts obtained from hexane, ethanol maceration and soxhlet extraction, Distilled water was freshly glass distilled (Genristo, Nottingham UK) in the laboratory.
Methodology
Preparation of agar plate
i. 38 grams of Mueller Hinton Agar powder were weighted.
ii. Powder was suspended in 1000 ml of distilled water in a conical flask.
iii. Conical flask was sealed with aluminium foil and put in the autoclave.
iv. Sterilized agar was poured into clean, empty petri dish for about half of the capacity of the petri dish. Process done in laminar air flow cabinet.
v. Wait for few minutes for the agar to solidify.
vi. Agar plates were stored in incubator at about 36°C.
Procedure
i. 500 μg /ml and 1000 μg/ml of different extracts were prepared by dissolve required quantity of extract in 10% Dimethyl sulfoxide (DMSO) or hexane.
ii. 1mg/ml of penicillin and 1mg/ml of ampicillin were used as a positive control; 10% DMSO or hexane was used as negative control.
iii. Bottom of prepared petri dish labeled with marker pen.
iv. 100 μm of bacterial strain was pipetted onto the surface of agar and spread equally by spreader.
v. By using borer (diameter 14 mm), holes were cut out from agar at 5 different spot corresponding to the label.
vi. By using micropipette, each hole was filled with respective materials. (penicillin, ampicillin, 500 μg/ml of extract, 1000 μg/ml of extract and 10% DMSO or hexane.
vii. Plate was covered and incubated in incubator at 37°C for 24 hours.
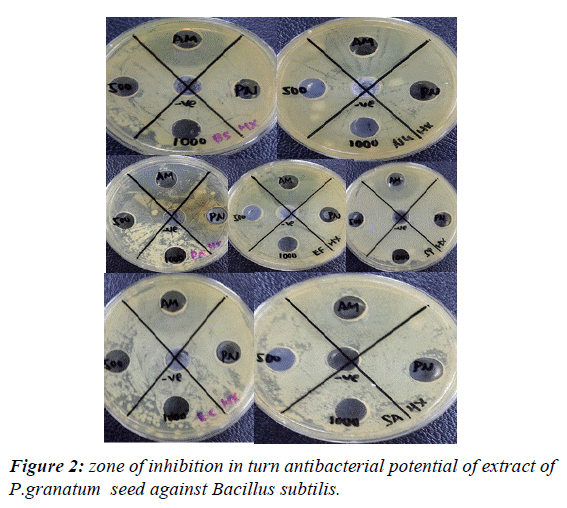
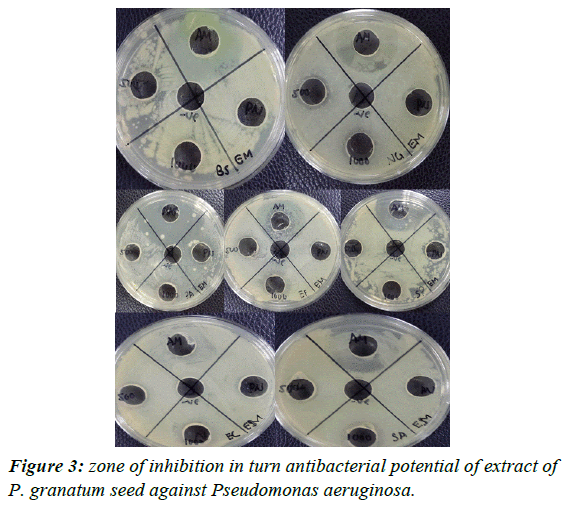
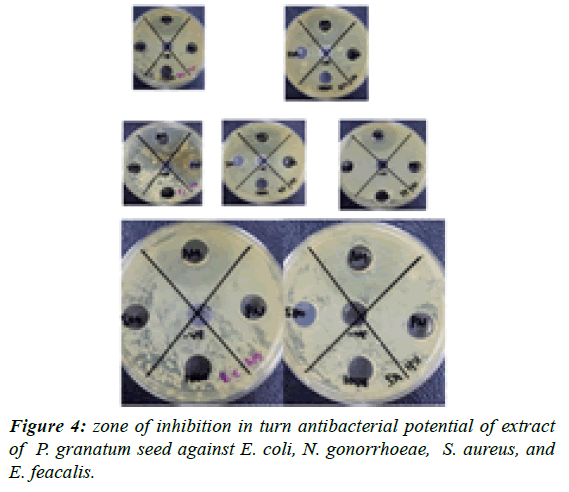
Results of antimicrobial activity: The experimental procedure was followed to determine the presence of zone of inhibition in turn antibacterial potential of extract of punica granatum seed against Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, Neisseria gonorrhoeae, Staphylococcus aureus, and Entero feacalis. The results (zone of inhibition) so obtained are presented in Table 5 and Figures 1-4.
| Microorganism | Concentrated | 500 | 1000 | Ampicillin | Penicillin | DMSO |
| Extract | mg/ml | mg/ml | ||||
| Bacillus subtilis | Ethanol soxhlet | 7 | 12 | 18 | _ | _ |
| Ethanol | _ | _ | 20 | _ | _ | |
| maceration | ||||||
| Hexane | _ | _ | 18 | _ | _ | |
| maceration | ||||||
| Neisseria | Ethanol soxhlet | 8 | 12 | 19 | _ | _ |
| gonorrhoeae | Ethanol | 3 | 2 | 16 | _ | _ |
| maceration | ||||||
| Hexane | _ | _ | 20 | _ | _ | |
| maceration | ||||||
| Pseudomonas | Ethanol soxhlet | 6 | 12 | 16 | _ | _ |
| aeruginosa | Ethanol | 2 | 16 | 20 | _ | _ |
| maceration | ||||||
| Hexane | _ | _ | 17 | _ | _ | |
| maceration | ||||||
| Enterococcus feacalis | Ethanol soxhlet | 6 | 10 | 16 | _ | _ |
| Ethanol | _ | _ | 17 | _ | _ | |
| maceration | ||||||
| Hexane | _ | _ | 11 | _ | _ | |
| maceration | ||||||
| Strepcoccus pneumonia | Ethanol soxhlet | 8 | 10 | 14 | _ | _ |
| Ethanol | _ | _ | 19 | _ | _ | |
| maceration | ||||||
| Hexane | _ | _ | 18 | _ | _ | |
| maceration | ||||||
| Eschericia coli | Ethanol soxhlet | 7 | 10 | 13 | _ | _ |
| Ethanol | _ | _ | 16 | _ | _ | |
| maceration | ||||||
| Hexane | _ | _ | 20 | _ | _ | |
| maceration | ||||||
| Staphylococcus | Ethanol soxhlet | 5 | 12 | 11 | _ | _ |
| aureus | Ethanol | 6 | 12 | 18 | _ | _ |
| maceration | ||||||
| Hexane | _ | _ | 10 | _ | _ | |
| maceration | ||||||
Table 5: Zone of inhibition (mm).
Determination of antioxidant activity
Chemicals: 1,1- diphenyl-2-picryl hydrazyl (DPPH) (Sisco Research Laboratories Pot Ltd), Butylated hydroxyltoluene (BHT) (Nacalai tesque, kyota, Japan), Ethanol (HmbG Chemicals), n-Hexane ( R&M Chemicals).
Methodology
DPPH (1, 1-diphenyl-2-picryl hydrazyl) Radical Scavenging Assay
i. The ethanol and hexane extracts were used to test the antioxidant activity.
ii. The extracts were prepared at six different concentrations of 10, 20, 40, 60, 80 and 100 μg/ml for both Soxhlet and maceration methods. The ethanol extract by Soxhlet method was labeled as EV1 for ES. The ethanol extract of maceration was labeled as EM. The hexane extract of maceration method were labeled as HX.
iii. The extracts were prepared by preparing the stock solution of 1mg/ml concentration by dissolving 10 mg of extract in 10 ml of ethanol or hexane respectively in a volumetric flask. The stock solution was serially diluted to obtain the concentration required for quantitative testing.
iv. From the stock solution (as prepared above), 0.1, 0.2, 0.4, 0.6, 0.8 and 1 ml volumes were taken in six properly labelled 10 ml volumetric flasks and the volume was made up to 10 ml to obtain a series of concentration.
v. Butylated hydroxyltoluene (BHT) was used as the standard for this quantitative study to determine the antioxidant activity. 10, 20, 40, 60, 80 and 100 μg/ml of BHT solutions was prepared in 10 ml volumetric flasks. Stock solution of 1 mg/ml of BHT was made in 10 ml of ethanol. 1, 0.8, 0.6, 0.4, 0.2 and 0.1 ml from the stock solution were placed in 10 ml volumetric flask. The volume in these volumetric flasks was made up to 10 ml of ethanol to obtain the series of concentration of standard BHT solution required.
vi. 0.3mm of alcoholic solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH) was prepared and 1 ml of the solution was added to 2.5 ml of plant extracts at different concentration to rinse test tubes. The mixtures were mixed well and were allowed to stand at room temperature in a dark place for 30 minutes.
vii. The absorbance was measured at 518 nm using a spectrophotometer at the end of 30 minutes.
viii. The same procedure was repeated for standard solution of BHT. All test solutions inclusive of the standard solutions of BHT was done in triplicates and the mean value was taken for analysis. The readings obtained were tabulated accordingly.
ix. The antioxidant activity was determined by using the following formula:

Results of Antioxidant Activity [21-25]
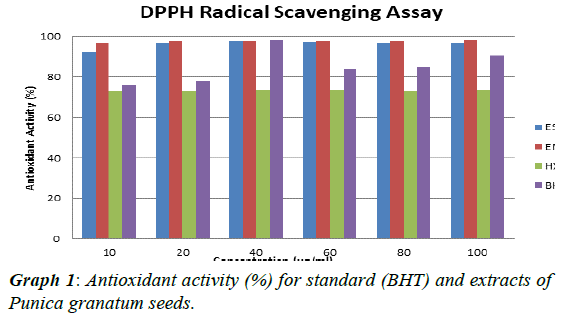
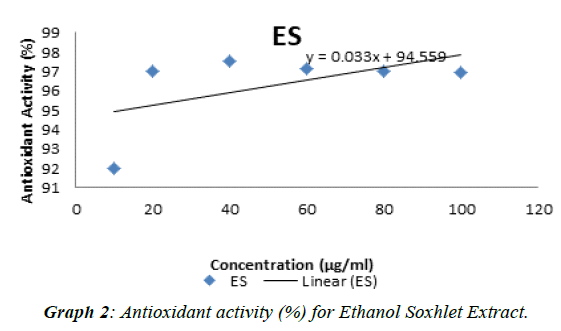
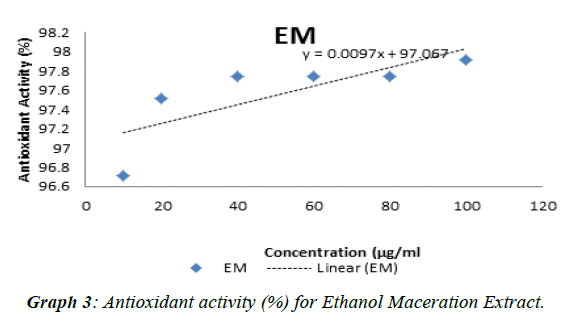
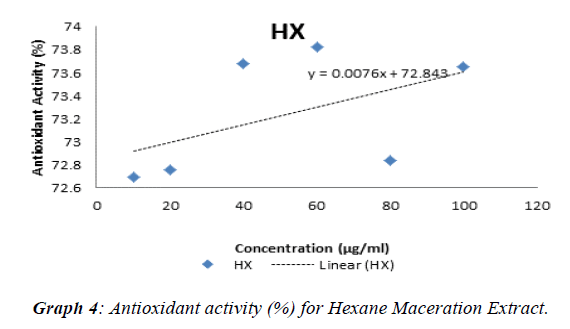
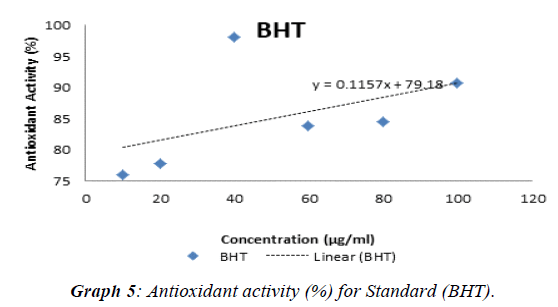
The experimental procedure was followed as per section 3.7, to determine antioxidant activity of extracts of Punica granatum seed. Antioxidant test was conducted with butylated hydroxytoluene (BHT) as standard. The results so obtained are presented in Table 6. Antioxidant activity (%) for standard (BHT) and extracts of Punica granatum seeds was calculated,tabulated in Table 7 and presented in (Graph 1). The antioxidant activity against concentration of standard and extracts of Punica granatum seed was plotted in Graphs 2, 3, 4, and 5. Inhibitory concentration (IC50), the half maximal inhibitory concentration for extracts of Punica granatum seeds were calculated by using the equations obtained from the graphs and tabulated in Table 8.
| Conc. | 10 | 20 | 40 | 60 | 80 | 100 |
| Sample | µg/ml | µg/ml | µg/ml | µg/ml | µg/ml | µg/ml |
| ES | 0.285 | 0.107 | 0.087 | 0.102 | 0.107 | 0.109 |
| EM | 0.117 | 0.088 | 0.08 | 0.08 | 0.08 | 0.074 |
| HX | 0.97 | 0.968 | 0.935 | 0.93 | 0.965 | 0.936 |
| BHT | 0.854 | 0.788 | 0.0681 | 0.573 | 0.551 | 0.33 |
Table 6: Absorbance value of standard and extracts of Punica granatum seeds at 518NM, Control (DPPH) absorbance=3.552
| Concentrated | 10 | 20 | 40 | 60 | 80 | 100 |
| Sample | µg/ml | µg/ml | µg/ml | µg/ml | µg/ml | µg/ml |
| ES | 91.98 | 96.99 | 97.55 | 97.13 | 96.99 | 96.93 |
| EM | 96.71 | 97.52 | 97.75 | 97.75 | 97.75 | 97.92 |
| HX | 72.69 | 72.75 | 73.68 | 73.82 | 72.83 | 73.65 |
| BHT | 75.96 | 77.82 | 98.08 | 83.87 | 84.49 | 90.71 |
Table 7: Antioxidant activity (%) for standard (BHT) and extracts of Punica granatum seeds.
| Extract / Standard | IC50 value (μg/mL) |
| ES | 1350.3 |
| EM | 4852.3 |
| HX | 3005.7 |
| BHT | 252.2 |
Table 8: IC50, the half maximal (50%) inhibitory concentration for extracts.
Discussion
Punica granatum is reported as a herbal medicine that is useful in treating various diseases and it is due to its antioxidant and antimicrobial activity. Therefore, this study was planned to investigate the Punica granatum seeds for antibacterial and antioxidant activity. In present study, the seeds of Punica granatum were collected and dried under shade for three days. The different extracts were investigated for their antimicrobial activity (using well diffusion method) to Escherichia coli, Bacillus subtilis, Neisseria gonorrhoeae, Staphylococcus aureus, Enterococcus feacalis and Pseudomonas aeruginosa. Penicillin and ampicillin were used as positive control in this study. Ethanol soxhlet (ES) extract showed inhibitory effect over growth of all the seven strains of bacteria such that zone of inhibition was detected. Ethanol maceration (EM)) extract only possessed antimicrobial activity against Neisseria gonorrhoeae, Pseudomonas aeruginosa and Staphylococcus aureus whereas hexane extract do not showed any antimicrobial activity. Penicillin as one of the positive control does not show any zone of inhibition. This may be due to resistance of these bacteria towards penicillin or spoilage of the prepared stock solution in the laboratory. The comparison of zone of inhibitions of different extracts (2-8 mm) and ampicillin (11-34 mm) against seven bacterial strains at concentration of 500 μg / ml, showed that antibacterial potential of punica granatum seed extracts was 6-47% of the antibacterial potential of ampicillin. The comparison of zone of inhibitions of different extracts (2-16 mm) and ampicillin (11-34 mm) against seven bacterial strains at concentration of 1000 μg / ml, showed that antibacterial potential of Punica granatum seed extracts was 10-68% of the antibacterial potential of ampicillin. For the antioxidant activity, DPPH radical scavenging assay was performed. IC50, the half maximal (50%) inhibitory concentration was measured in this study. Ethanol soxhlet (ES) extract showed the lowest IC50 value (1350.27 μg / ml) hexane extract (HM) showed IC50 value of 3005.66 μg / mL and ethanol maceration extract (EM) showed highest IC50 value (4852.26 μg / ml).
Conclusion
The seeds of Punica granatum has has shown substantial antibacterial and antioxidant activity. To have more insight into the mechanism of action of Punica granatum, further investigation on isolates of extracts should be done. It is concluded that the good antibacterial and antioxidant potential of Punica granatum seeds with ethanol soxhlet extraction.
Ethical Approval
The joint committee of School of Pharmaceutical Sciences, USM, Penang – Lam Wah Ee Hospital, Penang on clinical studies approved the protocol of this study with reference letter No. USM-HLWE/IEC/2011 (0016) on 30.06.2012. The study was also registered with National Medical Research Registry (NMRR) and was approved by Medical Research & Ethics Committee (MREC), National Medical Research Register Malaysia (NMRR Reference ID. 10124 (Nabila Perveen C/O Azmi Sarriff) on 14.10.2011.
Acknowledgements
This research work was sponsored by the Faculty of Pharmacy, AIMST University, Bedong, Kedah DA, Malaysia, which is highly appreciated.
Conflict of interest
The author declares no conflict of interest
References
- Morton JF. "Pomegranate, Punica granatum L. Fruits of Warm Climates. Purdue New Crops.2009.
- Andrew Tang Lonely P. The anar is native to the region around Iran and is eaten fresh and incorporated in a range of Persian dishes most famously in fesenjun, but also in ash-e-anar and in rich red ab anar 11-29.
- Hopf M. Domestication of plants in the old world: the origin and spread of cultivated plants in West Asia, Europe, and the Nile Valley. Oxford University 2000:171.
- Stover E, Mercure EW . "The pomegranate: a new look at the fruit of paradise". HortScience. 2007;42:1088-92.
- Jindal KK. Sharma RC. Recent trends in horticulture in the Himalayas. Indus Publishing. Bark of tree and rind of fruit is commonly used in Ayurveda.2004.
- Verotta, Luisella, Maria P, et al. Connecting Indian Wisdom and Western Science: Plant Usage for Nutrition and Health.
- Seeram, NP, Schulman, RN, Heber D, et al. Pomegranates: Ancient Roots to Modern Medicine. 2006.
- El-nemr SE, Ismail IA, Ragab M. Chemical composition of juice and seeds of pomegranate fruit.1990.
- Satheesh Bhandary, Vadish Bhat, Mahesh PB, et al. Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit and seeds. 2012
- Elbandy MA, Ashoush IS. Phytochemicals in Pomegranate Seeds and Their Effect as Hypolipidemic Agent in Hypercholesterolemic Rats. 2012.
- Illana Louise Pereira de Melo, Eliane Bonifácio Teixeira de Carvalho, Jorge MF, etal. Pomegranate Seed Oil. A Source of Punicic Acid 2014.
- Shay Yehoshua Schubert, Ishak Neeman, Ephraim PL, et al. Journal of Ethnopharmacology. 1999; 66: 11-
- Gómez-Caravaca AM, Verardo V, Toselli M, et al. Determination of the major phenolic compounds in pomegranate juices by HPLC−DAD−ESI-MS. Journal of Agricultural and Food Chemistry. 2013; 61:5328-37.
- Singh RP, Chidambara Murthy KN, Jayaprakasha GK, etal. "Studies on the Antioxidant Activity of Pomegranate Peel and Seed Extracts Using in Vitro Models". Journal of Agricultural and Food Chemistry. 2002; 50:81–6.
- Sheets M.D, DuBois M.L, Williamson J.G, Horticultural Sciences Department, J Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida Gainesville FL 32611.
- Hernández F, Melgarejo P, Tomás-Barberán FA, etal. "Evolution of juice anthocyanins during ripening of new selected pomegranate clones". European Food Research and Technology. 1999;210:39-42.
- Neslihan AK, Savas Bahçeci, Jale Acar, etal. Journal of Food Processing and Preservation. 2005; 29:357-68.
- Sharrif MM, Hamed Haddad Kashani, Chemical composition of the plant Punica granatum L.and its effect on heart and cancer. 2011.
- Jayaprakasha GK, Negi PS, Jena BS, etal. "Antimicrobial activities of pomegranate". ancient roots to modern medicine. 2006;168.
- Saad Sabbar Dahham, Ali MN, Hajera Tabassum, etal. Studies on Antibacterial and Antifungal Activity of Pomegranate 2010.
- Madrigal-Carballo S, Rodriguez G, Krueger CG, etal. Pomegranate supplements: Authenticity, antioxidant and polyphenol composition. 2009.
- Singh RP, Chidambara Murthy KN, Jayaprakasha GK, Studies on the Antioxidant Activity of Pomegranate Peel and Seed Extracts Using in Vitro Model. 2001.
- Singh RP, Chidambara Murthy KN, Jayaprakasha GK, "Studies on the Antioxidant Activity of Pomegranate Peel and Seed Extracts Using in Vitro Models". Journal of Agricultural and Food Chemistry.2002;50:81-6.
- Plumb GW, De Pascual-Teresa S, Santos-Buelga C, etal. "Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel". 2002; 7:41-6.
- Chidambara Murthy KN, Jayaprakasha GK, Singh RP, etal. "Studies on Antioxidant Activity of Pomegranate Peel Extract Using in Vivo Models". Journal of Agricultural and Food Chemistry. 2002; 50: 4791.