Research Paper - Otolaryngology Online Journal (2016) Volume 6, Issue 3
Repetitive Transcranial Magnetic Stimulation (Rtms) Treatment for Chronic Tinnitus: Effects of Active Stimulation on Study Participants who Initially Received Sham Rtms
- *Corresponding Author:
- Folmer RF
National Center for Rehabilitative Auditory Research, Portland VA Medical Center
3710 S.W. U.S. Veterans Hospital Road (NCRAR), Portland, U.S.A
Tel: 503-220-8262
E-mail: Robert.Folmer@va.gov
Received date: April 07, 2016; Accepted date: May 18, 2016; Published date: May 23, 2016
Abstract
In a recently published clinical trial, 32 tinnitus patients underwent 10 sessions of active 1 Hz repetitive transcranial magnetic stimulation (rTMS)-2000 pulses per session. The control group consisted of 32 tinnitus patients who underwent 10 sessions of placebo (or “sham”) 1 Hz rTMS. When participants completed all of the follow-up assessments (26 weeks after the last rTMS session), those in the “sham” rTMS group were informed of their assignment and invited to return to undergo 10 sessions of active rTMS. This article reports results for 17 clinical trial participants (13 males, 4 females) who initially received sham rTMS, then returned and underwent 10 sessions of active rTMS. The primary outcome measure was the Tinnitus Functional Index (TFI) questionnaire. Three of these participants were “responders” to placebo rTMS during the original clinical trial, with a mean decrease of 18.8 points on TFI post-treatment. None of these 3 participants exhibited significant changes in TFI score following 10 sessions of active rTMS in the present study. Of the remaining 14 participants, 6 were responders to active rTMS, with a mean decrease of 16.8 ± 8.3 TFI points post-treatment compared to baseline scores. However, the magnitude of this improvement was not maintained throughout the 26-week follow-up period (as it was in the original clinical trial). In this study, the mean TFI score for 6 treatment responders at the 26-week assessment was only 4.4 ± 7.8 points lower than their new baseline score. This result might be due to, 1) the smaller sample size in this study, or 2) reduced susceptibility to rTMS treatment in this group of participants.
Introduction
While tinnitus (the perception of ringing or other phantom sounds in the ears or head) is perceived by 10-15% of the adult population1, only 20% of people who perceive tinnitus consider it to be a “clinically significant” problem2. A large majority of people with chronic tinnitus also experience hearing loss or have damage to/degeneration of auditory structures, including those in the inner ear. Functional neural imaging studies have detected superfluous activity in brain regions (such as auditory cortex) of people who perceive tinnitus3-5. Apparently, damage to or degeneration of auditory structures sometimes results in patterns of aberrant neural activity that are responsible for generating the tinnitus perception. Because repetitive transcranial magnetic stimulation (rTMS) can suppress or alter neural activity, numerous researchers and clinicians have investigated rTMS as a treatment for tinnitus6.
We recently published results of a clinical trial in which 2000 pulses of 1 Hz rTMS were applied to the temporal lobe of tinnitus patients during each of 10 treatment sessions7. In this study, half of the participants received active rTMS and the other half received placebo (or “sham”) rTMS. The placebo coil was identical in appearance to the active coil and produced sounds and scalp sensations that were similar to those produced by the active coil. The manufacturer (Magstim Company Ltd) asserts that the placebo coil contains a metal plate that blocks much of the magnetic field it generates from affecting neural activity. Study participants were also randomized to receive rTMS on either the left or right side of their head. Results indicated that 18 of 32 participants (56%) in the active rTMS group and 7 of 32 participants (22%) in the sham rTMS group were responders to rTMS treatment. The difference in the percentage of responders to treatment in each group was statistically significant (p<0 .005). “Responders” to rTMS were defined as those participants who exhibited a reduction of >7 points on the Tinnitus Functional Index (TFI) questionnaire8 after their last (10th) rTMS session compared to baseline. For most treatment responders, improvements in tinnitus severity (as measured by the TFI) were sustained – or increased – throughout the 6-month followup period. When participants completed all of the follow-up assessments (26 weeks after the last rTMS session), those in the “sham” rTMS group were informed of their assignment and invited to return to undergo 10 sessions of active rTMS. This article reports results for clinical trial participants who initially received sham rTMS, then returned and underwent 10 sessions of active rTMS.
Methods
All procedures for recruitment, informed consent, and conduct of the study adhered to the requirements of the Institutional Review Board at VA Portland Medical Center, where the study was conducted between 2011-2015. For details about the original clinical trial, including rTMS
Administration parameters7, briefly subjects received 2000 pulses of 1 Hz rTMS therapy daily on 10 consecutive work days. The scalp target for rTMS was the temporal region overlying auditory cortex9. For this study, participants received rTMS on the same side of the head (either left or right) as they did for the original clinical trial, when they received sham rTMS. RTMS intensity was either 60% of the Magstim system capacity or 110% of participants’ resting motor threshold (rMT), whichever was lower. Stimulation intensity could be reduced further if participants experienced pain or discomfort during rTMS sessions.
All subjects wore foam ear plugs (which were inserted by the study audiologist) during rTMS sessions. Ear plugs were used for 3 reasons: 1) to attenuate the loud clicking sound of rTMS itself in order to maximize subjects’ comfort during sessions; 2) to minimize the effects of this sound on subjects’ perception of tinnitus; 3) to protect subjects’ hearing. Pure tone audiograms were recorded for all subjects in a sound-attenuated booth before the first and after their last (10th) rTMS session.
Outcomes were measured prior to the start of treatment (baseline) and immediately after the last (10th) rTMS session. Follow-up evaluations were conducted 26 weeks after the last treatment session. The primary outcome measure was the TFI questionnaire (Meikle et al.). Participants also completed a visual numerical (0-to-10) scale (VNS) for self-rated tinnitus loudness (shown below) before and after each rTMS session and at the 26- week follow up appointment.
On the scale below, please draw a vertical line to indicate the loudness of your tinnitus at this moment
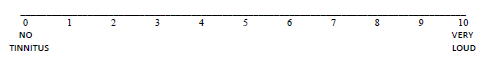
Participants were paid $20 for each baseline assessment, rTMS session, and follow-up assessment they attended.
Results
Seventeen participants (13 males, 4 females) who initially received sham rTMS during the original clinical trial returned and received 10 sessions of active rTMS during this study. (Table 1) lists group data for these 17 participants. Table 2 contains data for each of the individual study participants. Pure tone audiograms – recorded for all subjects before the first and after their last (10th) rTMS session– showed that rTMS did not have any significant effects on subjects’ hearing thresholds.
| Number of males | 13 |
| Mean age (years) ± SD | 61.9± 8.3 |
| Duration of tinnitus (years) 1-2 3-5 6-10 11-20 >20 |
2 subjects 1 subjects 1 subjects 4 subjects 9 subjects |
| TFI score (baseline) – Mean ± SD | 40.4 ± 21.0 |
| VNS tinnitus loudness (baseline) – Mean ± SD | 7.1 ± 1.4 |
| Resting motor threshold (rMT) % – Mean ± SD | 60.6 ± 4.2 |
| TMS intensity % – Mean ± SD | 57.1 ± 6.3 |
Table 1: Group data from 17 study participants.
During rTMS sessions, some subjects experienced involuntary jaw movement or twitching; eye twitching; and mild discomfort from TMS pulses delivered to the temporal region of their scalp. Reducing the intensity of rTMS stimulation was requested by five participants (numbers 7, 10, 12, 15 and 17). After the stimulation intensity was reduced (by 5-10%), all of these subjects then completed 10 sessions of rTMS with few – if any – complaints of discomfort.
(Table 2) the change (from baseline) in TFI score for all study participants. In the original clinical trial – when these 17 participants underwent 10 sessions of placebo rTMS – three subjects (numbers 3, 9, 17) exhibited significant improvement in TFI score post-TMS compared to their baseline score. The mean decrease in TFI score for these 3 participants was 18.8 points, or 33% below their baseline TFI score (mean=57.5). When these participants returned for active rTMS, all of their new baseline TFI scores remained significantly lower (mean=34.8) than their original baseline TFI scores. None of the other participants in this study exhibited significant improvement in TFI score after they received placebo rTMS during the original clinical trial.
| Participant Number | Gender | Age (Years) |
Tinnitus Duration (Years) |
Tinn Percept |
Side of rTMS | Original Baseline TFI score |
ΔTFI post sham rTMS |
New Baseline TFI score |
ΔTFI post active rTMS | ΔTFI 26 weeks post rTMS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 69 | 11-20 | Center | R | 91.6 | +1.6 | 68.4 | +5.6 | -9.6* |
| 2 | M | 59 | 6-10 | Center | R | 26.0 | -2.8 | 26.8 | -10.4* | -13.6* |
| 3 | F | 57 | 1-2 | L>R | L | 63.2 | -25.6* | 24.8 | -1.6 | -8.8* |
| 4 | M | 57 | 11-20 | L>R | L | 56.8 | -4.8 | 43.2 | +10.8 | +0.8 |
| 5 | M | 64 | >20 | L>R | L | 65.6 | +0.8 | 55.2 | -32.8* | -4.8 |
| 6 | M | 52 | 3-5 | R>L | L | 63.2 | -2.0 | 50.0 | +8.4 | +8.4 |
| 7 | M | 63 | >20 | L>R | L | 66.4 | +15.6 | 86.8 | +3.6 | -1.2 |
| 8 | F | 56 | 11-20 | L>R | L | 16.4 | +7.2 | 18.4 | -3.2 | -4.8 |
| 9 | M | 41 | >20 | L>R | L | 25.2 | -11.6* | 12.8 | -3.2 | +2.4 |
| 10 | M | 72 | >20 | R>L | R | 32.4 | +3.6 | 47.6 | -16.4* | +2.8 |
| 11 | M | 59 | 11-20 | R>L | R | 46.0 | +3.2 | 17.2 | +12.8 | 0 |
| 12 | M | 64 | >20 | L>R | R | 36.8 | +3.6 | 26.4 | -8.8* | +7.6 |
| 13 | F | 71 | >20 | L>R | L | 68.4 | -3.2 | 55.2 | -13.6* | -7.6* |
| 14 | F | 66 | >20 | Center | R | 22.4 | +2.8 | 24.8 | +5.2 | -5.6 |
| 15 | M | 78 | >20 | Center | L | 16.8 | +14.4 | 42.4 | -18.8* | -10.8* |
| 16 | M | 63 | >20 | R>L | R | 23.2 | -6.4 | 20.4 | -4.8 | -13.2* |
| 17 | M | 61 | 1-2 | R>L | L | 84.0 | -19.2* | 66.8 | -4.4 | +5.2 |
| Mean | 61.9 | 47.3 | -1.3 | 40.4 | -4.2 | -3.1 | ||||
| SD | 8.3 | 23.9 | 10.3 | 21.0 | 11.7 | 6.9 | ||||
| p-value | 0.61 | 0.16 | 0.08 | |||||||
| Effect size | 0.13 | 0.36 | 0.45 |
Table 2: Individual data from 17 study participants.
Results of active rTMS on TFI scores are also shown in (Table 2). For the group of 17 participants, the mean change in TFI score post-TMS (compared to their new baseline TFI score) was -4.2 ± 11.7 points – an effect size of 0.36. In this group, 6 participants were classified as “responders” to rTMS (exhibited reductions in post-TMS TFI score >7 points compared to their new baseline score). Five of these six responders to active rTMS treatment experienced tinnitus for more than 20 years. None of the three participants who responded to placebo rTMS in the original clinical trial (numbers 3, 9, 17) exhibited significant post-treatment changes in TFI score after 10 sessions of active rTMS. (Table 3) a tabulation of location of tinnitus perception vs. side of rTMS delivery for 17 study participants. As in the original clinical trial, there is no clear pattern indicating an optimal side of rTMS delivery for a particular laterality of tinnitus perception (Table 4). The mean change (from baseline) in TFI scores for these 6 “Responders” to active rTMS. While the magnitude of post-TMS improvement in TFI score for these 6 responders (mean reduction=-16.8 ± 8.3 points) was similar to that for 18 rTMS responders in the original clinical trial (mean reduction=-14.1 ± 6.0 points), the improvement was not maintained as well in the current study. As shown in Table 3, the mean TFI score for 6 treatment responders at the 26-week assessment was only 4.4 ± 7.8 points lower than their new baseline score. By contrast, the mean improvement in TFI score for treatment responders at the 26-week assessment in the original clinical trial was -19.7 ± 14.7 points. Table 5 shows that TFI scores did not change significantly for 11 non-responders to active rTMS in the current study.
| Side of rTMS | ||
|---|---|---|
| Left | Right | |
| Side of Tinnitus Perception | ||
| L>R (n=8) | 7 (2 responders) |
1 (1 responder) |
| Center (n=4) | 1 (1 responder) |
3 (1 responder) |
| R>L (n=5) | 2 (0 responders) |
3 (1 responder) |
Table 3: Side of Tinnitus Perception vs. Side of rTMS delivery to head.
| Change in TFI Score Compared to Baseline | ||
|---|---|---|
| Baseline TFI score (mean ± SD) |
Immediately Post-TMS |
26 weeks Post-TMS |
| 42.3 ± 12.5 | -16.8 ± 8.3 | -4.4 ± 7.8 |
| P-value → | 0.004 | 0.226 |
| Effect Size → | 2.02 | 0.56 |
Table 4: Mean change (from baseline) in TFI score for 6 “Responders” to rTMS.
| Change in TFI Score Compared to Baseline | ||
|---|---|---|
| Baseline TFI score (mean ± SD) |
Immediately Post-TMS |
26 weeks Post-TMS |
| 39.4 ± 24.7 | +2.7 ± 6.3 | -2.4 ± 6.5 |
| P-value → | 0.186 | 0.249 |
| Effect Size → | 0.43 (increase) |
0.37 (decrease) |
Table 5: Mean change (from baseline) in TFI score for 11 “Non-Responders” to rTMS.
(Tables 6-8) show changes in participants’ selfrated tinnitus loudness (VNS scale) following 10 sessions of active rTMS. The group of participants as a whole exhibited a reduction in VNS tinnitus loudness (mean=-0.82 ± 1.23 points on the 0-to-10 scale compared to baseline) immediately after completing 10 sessions of active rTMS (Table 6). However, at the 26-week assessment, the mean reduction in VNS tinnitus loudness was only -0.43 ± 1.20 points compared to baseline values. Table 7 shows that 6 rTMS treatment responders exhibited significant reductions in VNS tinnitus loudness (mean=-1.40 ± 0.60 points on the 0-to-10 scale compared to baseline) immediately after completing 10 sessions of r TMS. However, at the 26-week assessment, the mean reduction in VNS tinnitus loudness for the 6 responders was only -0.87 ± 1.31 points compared to their baseline values. Table 8 shows that 11 “Non- Responders” to rTMS treatment exhibited smaller, less significant reductions in VNS tinnitus loudness at both post-treatment assessments compared to baseline.
| Change in VNS Tinnitus Loudness Compared to Baseline | ||
|---|---|---|
| Baseline VNS loudness score (mean ± SD) | ImmediatelPost-TMS | 26 weeks Post-TMS |
| 7.1 ± 1.4 | -0.82 ± 1.23 | -0.43 ± 1.20 |
| P-value → | 0.014 | 0.159 |
| Effect Size → | 0.67 | 0.36 |
Table 6: Mean change (from baseline) in VNS Tinnitus Loudness for 17 study participants.
| Change in VNS Tinnitus Loudness Compared to Baseline | ||
|---|---|---|
| Baseline VNS loudness score (mean ± SD) | Immediately Post-TMS |
26 weeks Post-TMS |
| 6.5 ± 1.1 | -1.40 ± 0.60 | -0.87 ± 1.31 |
| P-value → | 0.002 | 0.165 |
| Effect Size → | 2.33 | 0.66 |
Table 7: Mean change (from baseline) in VNS Tinnitus Loudness for 6 “Responders” to rTMS.
| Change in VNS Tinnitus Loudness Compared to Baseline | ||
|---|---|---|
| Baseline VNS loudness score (mean ± SD) | Immediately Post-TMS |
26 weeks Post-TMS |
| 7.5 ± 1.4 | -0.50 ± 1.38 | -0.19± 1.05 |
| P-value → | 0.257 | 0.562 |
| Effect Size → | 0.36 | 0.18 |
Table 8: Mean change (from baseline) in VNS Tinnitus Loudness for 11 “Non-Responders” to rTMS.
Discussion
In this follow-up study, 6 of 14 tinnitus patients who did not respond to sham rTMS in a previous clinical trial7 exhibited significant reductions in tinnitus severity (as measured by the TFI questionnaire) after undergoing 10 sessions of active 1 Hz rTMS to the temporal lobe. Three other tinnitus patients who did respond to sham rTMS in the previous trial did not exhibit additional significant changes in TFI score after undergoing 10 sessions of active rTMS in this study. Why did 3 participants exhibit significant reductions in tinnitus severity after undergoing 10 sessions of sham rTMS in the original trial? At least 2 explanations are possible: 1) this might have been a true “placebo effect” of the procedure; 2) because the sham coil delivered a significant amount of electrical/mechanical energy to participants’ heads during stimulation, it might not have been a completely inert placebo. It is possible that the sham coil we used had a physiological effect on the auditory system/tinnitus of some participants.
Although the responders’ reduction in TFI score following active rTMS in this study was Similar to that exhibited by treatment responders in the original trial7 the Magnitude of improvement in tinnitus severity was not sustained at the 26-week follow-up Assessment (as it was for the original trial). This result might be due to, 1) the smaller sample size in this study, or 2) reduced susceptibility to rTMS treatment in this group of participants. In this study, changes in participants’ self-rated tinnitus loudness (VNS scale) following sessions of active rTMS tended to follow the pattern of VNS loudness results in the original clinical trial Folmer et al. In both studies, the VNS loudness measure was less sensitive to treatment-related change compared to the TFI questionnaire. These results are in agreement with Meikle et al. who reported that effect sizes for TFI were greater than those obtained using a visual analog scale (VAS) for tinnitus loudness. It is possible that the rTMS protocol we used had a more significant effect on tinnitus severity (as measured by the TFI) than it did on tinnitus perception/ loudness. One explanation for this: perhaps 1 Hz rTMS delivered to the temporal lobe altered or disrupted neural activity between auditory cortex and limbic regions (e.g. parahippocampus) or frontal cortex that facilitates adverse reactions to tinnitus. The rTMS procedures used in this study might have had relatively less impact on the neural generators of tinnitus perception.
Conclusions
This study provides additional evidence for the efficacy of 1 Hz rTMS as a treatment for tinnitus. The fact that five of six responders to active rTMS treatment experienced tinnitus for more than 20 years indicates that these treatment protocols are effective for some patients with long-duration tinnitus as well as those with shorter-duration symptomology. Larger, multi-site clinical trials are needed to refine rTMS protocols for tinnitus and to further address the issue of rTMS laterality vs. laterality of tinnitus perception.
However, we do not believe that rTMS should be viewed as a replacement for effective tinnitus management strategies that are available now10- 13. Instead, rTMS could provide a viable option for patients who do not respond favorably to other treatments.
Acknowledgements
This research was supported by a grant from the U.S. Department of Veterans Affairs Rehabilitation Research and Development (RR&D) Service. Additional support was provided by the VA National Center for Rehabilitative Auditory Research at VA Portland Medical Center.
References
- Hoffman HJ, Reed GW (2004)Epidemiology of tinnitus. In: J.B. Snow JB (Edn.), Tinnitus: Theory and Management (pp: 16-41). Lewiston, NY: BC Decker Inc.
- Henry JA, Zaugg TL, Myers PJ, Kendall CJ, Turbin MB (2009)Principles and application of educational counseling used in progressive audiologic tinnitus management. Noise Health 11: 33-48.
- Arnold W, Bartenstein P, Oestreicher E, Romer W, Schwaiger M (1996) Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL 58: 195-199.
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wach DS, et al. (1998) the functional neuroanatomy of tinnitus: evidence for limbic system links and neuroplasticity. Neurology50: 114-120.
- Folmer RL (2007) Lateralization of Neural Activity Associated with Tinnitus. Neuroradiology 49: 689-691.
- Theodoroff SM, Folmer RF (2013) Repetitive Transcranial Magnetic Stimulation as a Treatment for Chronic Tinnitus: A Critical Review. Otol Neurotol 34: 199-208.
- Folmer RL, Theodoroff SM, Casiana L, Shi YB, Griest SE, et al. (2015) Repetitive Transcranial Magnetic Stimulation Treatment for Chronic Tinnitus: A Randomized Clinical Trial. JAMA Otolaryngology-Head & Neck Surgery 141: 716-722.
- MeikleMB, Henry JA,Griest SE, Stewart BJ, Abrams HB, et al. (2012) The Tinnitus Functional Index: A new clinical measure for chronic, intrusive tinnitus. Ear & Hearing33: 153-176.
- Langguth B,Zowe M, Landgrebe M (2006) Transcranial Magnetic Stimulation for the treatment of tinnitus: a new coil positioning method and first results. Brain Topogr 18: 241-247.
- Andersson G, Lyttkens L (1999) A meta-analytic review of psychological treatments for tinnitus.Br J Audiol 33: 201-210.
- CimaRFF, Andersson G, Schmidt C, Henry J (2104) Cognitive-behavioral therapy for tinnitus: A review of literature. JAAA 25: 29-61.
- FolmerRL, Carroll JR (2006) Long-term effectiveness of ear-level devices for tinnitus.Otolaryngology - Head and Neck Surgery 134: 132-137.
- HenryJA, Zaugg TL, Myers PJ, Schechter MA (2008) Using therapeutic sound with progressive audiologic tinnitus management. Trends in Amplification 12: 188-209.