Research Article - International Journal of Pure and Applied Zoology (2016) Volume 4, Issue 2
Histological Organization And Ultr-Structures Of The Apical Surface Of The Olfactory Epithelium Of A Carp, Labeo Bata (Hamilton)
| Ishita Samajdar and Dipak Kumar Mandal* Department of Zoology, Centre for Advance Studies, Visva-Bharati University, Santiniketan-731235, West Bengal, India |
| Corresponding Author: Dipak Kumar Mandal E-mail: dkmandal.vb@gmail.com |
| Received 08th December 2015; Accepted 22nd January 2016; Published 02nd February 2016 |
Abstract
The olfactory organ being a major chemoreceptor organ in fish plays a significant role in searching food and mate, predator avoidance and reproduction. Structural organization of the olfactory shows variations among different fishes due to their adaptation to various habits and habitats. This study investigates the histological organization, histocheical nature and ultrastructures (SEM study) of the olfactory organ of Labeo bata (Hamilton). The peripheral end of the organ, olfactory rosette comprising 46-48 lamellae is provided with a large surface area of neurosensory epithelium and is placed on the floor of each nasal chamber. The organ communicates to the external environment through inlet and outlet openings of nasal aperture separated by a concave skin flap which facilitates water flow through nasal chamber. Two layered epithelium covering each lamella consists of sensory and non-sensory cells. Sensory cells cover lateral sides while non-sensory cells cover median ridge and outer edges of lamella. Sensory epithelium possesses both of the ciliated and microvillous olfactory receptor cells (cORC and mORC) along with supporting cells, basal cells and ciliated non-sensory cells. Histochemical localization revealed that mucous cells contain acidic and neutral mucin and distributed throughout the epithelium. Scanning Electron Microscopic (SEM) observations disclosed that the narrow dendrite ends of ORCs possess either 5-6 cilia or many microvilli on their apical surfaces. Broad apical surfaces of stratified non-sensory cells are provided with microridges. Functional significance of the different olfactory epithelial cells and fine structures on their apical surfaces are discussed. This study reveals that the olfactory organ of L. bata is well organized and seems to be efficient.
Keywords |
||||||||||
| Labeo bata; Histology; Histochemistry; SEM; Olfactory epithelium. | ||||||||||
INTRODUCTION |
||||||||||
| Olfaction is the major sensory modality of fish to perceive chemical signals for food searching, predator avoidance and reproductive activities (Hara, 1992). Morpho-anatomical features of the olfactory organ show variations among fishes due to their adaptation to different habit and habitats (Zeiske et al., 1992). There are number of studies on its histology (Zeiske et al., 1987; Hara and Zeilinski, 1989; Hara, 1993; Mandal et al., 2005, Baile et al., 2008) and electron microscopic features in different fish species (Bandyopadhyay and Datta, 1998; Fisher et al., 1984; Hansen and Finger, 2000; Mana and Kawamura, 2002) and that reveal variation in lamellar arrangement and distribution of sensory and non-sensory areas. Distribution patterns of sensory epithelium have been reported as continuous, discontinuous and scattered in islets. Olfactory receptor cells bearing either cilia or microvilli on their apical surfaces are common in teleosts (Zeiske et al., 1987, Mandal et al., 2005, Zeiske et al., 1979; Thommesen, 1983; Frabman, 2000; Ghosh and Chakrabarti, 2009) and a third type of sensory cell, crypt ORC has been reported in some fishes (Hansen, 2000). Labeo bata is an important freshwater food fish in India belongs to the family Cyprinidae. It is benthopelagic and herbivorous fish and suitable species for freshwater aquaculture. There are many studies on the olfactory organ of cyprinidae (Hansen et al., 2003; Ojha and Kapoor, 1973; Bhute and Baile, 2006; Chakrabarti and Ghosh, 2010a, Ghosh and Chakrabarti, 2011). However, detailed study on the histological organizations and topological structures of the olfactory epithelium of Labeo bata is lacking. Accordingly, the present study aimed to describe the tissue organizations and ultra-structures on the apical surfaces of different cells of the olfactory epithelium of L. bata through histological, histochemical and scanning electron microscopic analysis. | ||||||||||
MATERIALS AND METHODS |
||||||||||
Specimen procurement and tissue collection |
||||||||||
| Twenty specimen of Labeo bata (length 18.6 to 19.8 cm and weight 71.4 to 80.9 g) were procured from a fish pond at Santiniketan, West Bengal, India (Lat. 23°14´N long 87°51´E) and brought to the laboratory. Fish were anaesthetized with tricaine methonesulphonate (MS 222; Sigma chemical co.) solution (100 mg/L) and sacrificed following the guideline of the departmental animal ethics committee. The olfactory tissue was dissected out under a stereo-binocular microscope and immediately processed for the histological and Scanning Electron Microscopic studies. | ||||||||||
| Histological preparation: Olfactory tissues were fixed in aqueous Bouin’s fixative for a period of 20 hours and washed repeatedly with 70% ethanol to remove picric acid. The tissues were dehydrated through upgraded series of ethanol, cleared with benzene. The tissues were infiltrated with paraffin wax of 56-58°C under a thermostat vacuum paraffin embedding bath for 1 hour and embedded in paraffin blocks. Paraffin blocks of the tissue were sectioned at 4 μm thicknesses using a rotary microtome (Weswox, India, model 1090A). Tissue sections were stretched on Mayer’s albuminised glass slides, deparaffinised and stained with Mayer’s haematoxylin and 1% eosin stain. Slides were examined under Olympus BX52 compound microscope and photographic images were obtained. Thickness of epithelium, central core and size of different cells were measured using an ocular and stage micrometer (Erma, Japan) and data were represented as mean ± standard error. | ||||||||||
| Histochemical preparations: Boiun’s fixed and paraffin embedded olfactory tissues were sectioned at 10 μm thickness for different histochemical observations, such as periodic acid-schiff (PAS) (McManus, 1946), combined periodic acid schiff and alcian blue (PAS-AB) (Mowry, 1956) techniques to localize acid and neutral mucin. Silver impregnation technique (Marsland et al., 1954) was adopted for the detection of axonal processes of the olfactory receptor neurons. | ||||||||||
| Tissue preparation for scanning electron microscopy (SEM): After perfusion in vivo with 2.5% glutaraldehyde for 20 minutes, olfactory rosettes were obtained by careful dissection. Tissues were rinsed repeatedly in 1% tween 40 solutions and fixed in 2.5% glutaraldehyde solution for 24 h at 4°C. Tissues were washed in same buffer and post-fixed with 1% osmium tetraoxide (OsO4) for 2 hours. Fixed tissues were washed and dehydrated in ascending grades of ethanol followed by acetone and isoamyl acetate. Tissues were then dried by critical point drying technique with liquid carbon dioxide in a critical point drier (Hitachi 8CP2). Dried tissues were mounted on metal stub, coated with platinum (16 nm thick) using sputter coater (Quarum Q150tes) and examined under a Zeiss EVO18 Scanning Electron Microscope. | ||||||||||
RESULTS |
||||||||||
Morphology |
||||||||||
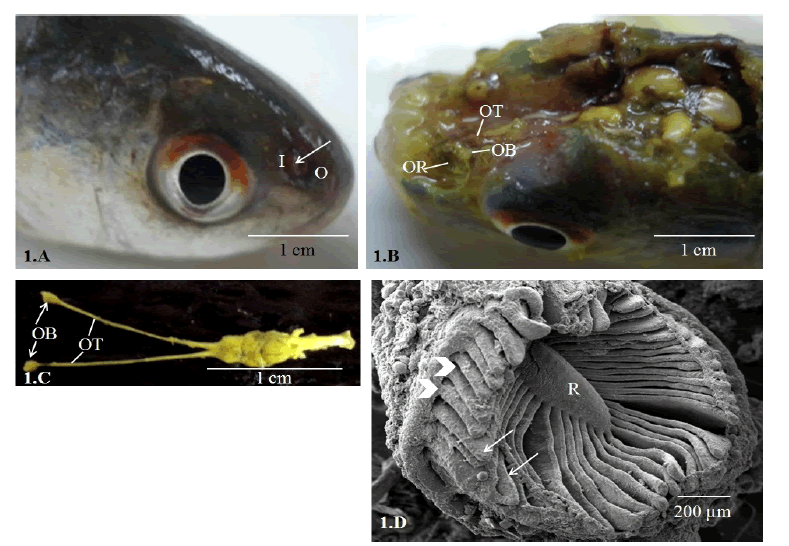
| Paired olfactory organ of Labeo bata are located on the floor of nasal chambers. Nasal chambers are situated dorsolaterally on the snout and open to the external environment through inlet and outlet channels separated by a concave membranous skin flap (Fig. 1.A). The major components of the pedunculate type olfactory organ of this fish are the olfactory rosette (OR), short olfactory nerve, olfactory bulb and a long olfactory tract connecting the bulb to the forebrain (Figs 1.B, 1.C). The olfactory rosette is oval in shape and comprises of 46-48 lamellae (OL) that radiated from a median ridge. The outer edges of the lamellae are attached to the wall of nasal chamber while their inner free margins are extended into linguiform processes (Fig. 1.D). | ||||||||||
Histology |
||||||||||
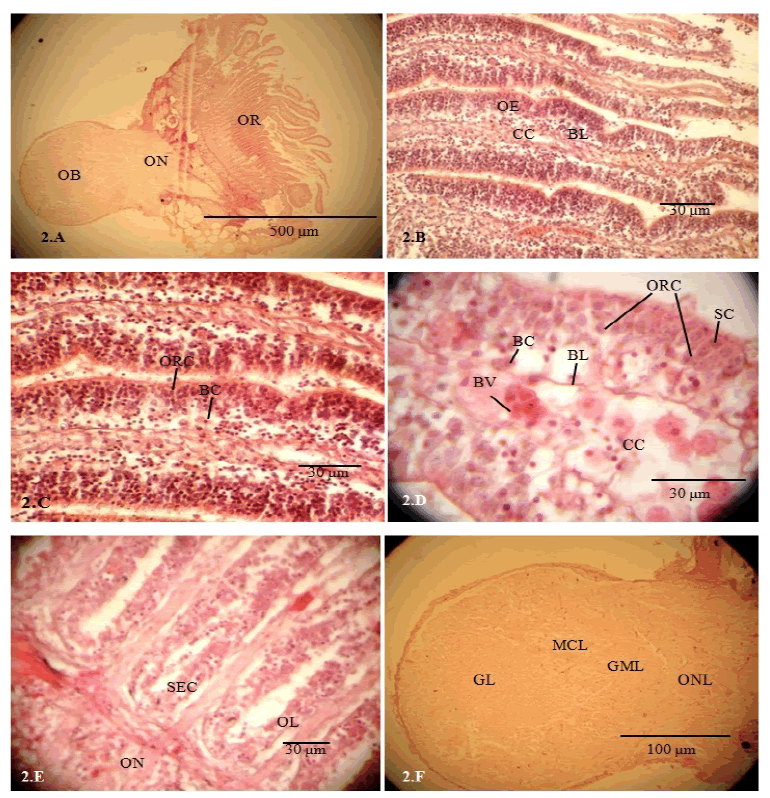
| Olfactory lamellae are formed due to folding of the epithelium and lamellae are arranged to form a cup shaped and oval olfactory rosette (OR). Each olfactory rosette comprises of 46-48 lamellae and is connected to the olfactory bulb by a short olfactory nerve (ON) (Fig. 2.A). Each lamella has two layers of epithelium that encloses a connective tissue layer, central core (Fig. 2.B). The central core (46.8 to 51.3 μm thick) consists of connective tissues, blood vessels and nerve fascicles (Figs 2.C, 2.D). The central core is separated from the epithelium by basement membrane. The epithelium shows unequal thicknesses varying from 31.46 ± 1.3 to 36.4 ± 1.2 μm. Non-sensory areas consist of stratified epithelium covering median raphe, inner and outer margins of the lamella. Sensory area consists of pseudostratified epithelium covering lateral sides of the lamella. Sensory epithelium is lined by ciliated and microvillous olfactory receptor cells (ORC), supporting cells, basal cells and ciliated non-sensory cells. ORCs are columnar (14.12-24.37 μm apical surface to cell body) that are extended from the epithelium surface to basal lamina. The round cell bodies with deeply stained nucleus are observed at different levels of the epithelium. The supporting cells (SC) are broad and columnar in shape (10.25-12.55 μm) with prominent nucleus located in more superficial level in the epithelium and these cells located in close association to the ORCs (Fig. 2.D). The basal cells (3.72-5.89 μm in diameter) are small, round with prominent central nucleus, situated at the basal part of the epithelium (Figs 2.C, 2.D). Basal cells (BC) are of two types, globular basal cells and horizontal basal cells which are distributed throughout the base of the epithelium (Figs 2.C, 2.D). Axonal processes of ORCs aggregate in the central core as nerve fascicles and finally form a short ON (Fig. 2E). Mucous cells (MC) (6.85-7.12 μm) are found all over the epithelium. The olfactory bulb is round in shape and connected to the olfactory rosette by a very short olfactory nerve (Fig. 2.A). Four distinct layers in the olfactory bulb are recognized from the periphery to center as nerve layer (NL), glomerular layer (GL), mitral cell layer (MCL) and granular cell layer (GCL) (Fig. 2.F). | ||||||||||
Histochemistry |
||||||||||
| Histochemical localization of mucopolysaccharide is evident through PAS positive reactions in the basement membrane and epithelium surface (Fig. 3.A). Mucous cells exhibit purple bluish colour in PAS-AB reaction which confirms the presence of acid and neutral mucin (Fig. 3.B). Supporting cells are PAS-AB positive at their apical surfaces. Olfactory receptor cells and basal cells are found negative to PAS-AB reaction (Figs 3.A, 3.B). | ||||||||||
| The apical surface of the epithelium, axonal processes and nerve fascicles in the central core and olfactory nerve show positive reaction with deep brown colour in silver staining (Fig. 3.C). The basement membrane and basal cells show negative reaction. ORCs and their axonal processes are detected in the mid -lateral part of the lamella (Fig. 3.D). | ||||||||||
Ultra-structure of the apical surface of the olfactory epithelium |
||||||||||
| Scanning electron microscopy reveals that 46-48 lamellae radiate from the median ridge and form a round cup-shaped olfactory rosette. The inner edge of each lamella is extended as a linguiform process (Fig. 4.A). The sensory epithelium covers the mid-lateral faces of the lamella while the nonsensory epithelium covers the edges of the lamella and median ridge (Fig. 4.B). The non-sensory stratified epithelial cells show polyhedral apical surfaces provided with zigzag pattern of microridges (Figs 4.C, 4.D, 4.E). A row of nonsensory ciliated cells is arranged along the free edge of lamella. The transitional zone of sensory and non-sensory epithelium is recognizable in the margin of lamella where both of the ORCs and non-sensory stratified epithelial cells (Fig.4.F) are found. | ||||||||||
| Sensory epithelium covering the mid-lateral faces of lamella (Fig. 5.A) comprises of ciliated ORC and microvillous ORC along with supporting cells and ciliated non-sensory cells. The narrow dendrite end of the ciliated ORC appears as olfactory knob consisting of 5-6 cilia while the broad apical surfaces of microvillous ORC possess many microvilli (Fig. 5.B, 5.F). The ciliated non-sensory cells in the sensory epithelium consist of tufts of cilia (Figs 5.C, 5.D). The sensory epithelium in the basal part of lamella is devoid of ciliated non-sensory cells (Fig. 5.E). Supporting cells possess broad apical surfaces with many short cilia and these cells are arranged around ORC (Fig. 5.F). Mucous cell openings are found throughout the epithelium. | ||||||||||
DISCUSSION |
||||||||||
| The first step of olfaction is to bring the odorant molecules into the nasal chamber and for that, fish adopt different nasal ventilation mechanism (Døving et al., 1977 and Belanger et al., 2003). There are many options for the nasal ventilation in fish such as entry of water during forward motion of fish or by hydraulic pumping of the olfactory sac or by continuous beating of cilia of the ciliated non-sensory cells (Hara, 1993). In L. bata, a concave skin flap between inlet and outlet channels provides a mechanical advantage to deflect water flow into the chamber during forward motion of fish. However, during static condition the fish may employ other mechanisms such as beating of non-sensory cilia. Like other bony fishes the olfactory mucosa of L. bata is extensively folded to accommodate large surface area in the limited space of nasal chamber and forms 46-48 lamellae. Many authors suggest that the extensive surface area of olfactory mucosa enhances the sensitivity and efficiency of the organ (Zeiske et al., 1976). However, this simple correlation hardly exists in fish because ORCs are not consistent in distribution and density (Yamamoto, 1982). Lamellae number and arrangement vary considerably among fish with their body shapes and habitats (Yamamoto, 1982; Zeiske et al., 1992). Like other cyprinid fish as for example Labeo rohita (Bhute and Baile, 2006) and Catla catla (Chakrabarti and Ghosh, 2010b), L. bata also has an oval and multi-lamellar olfactory rosette. The lamellae number is increased with age but after certain state of growth the number becomes constant for a fish species (Zeiske et al., 1992). The oval shaped olfactory organ belongs to eye nose fish (Teichmann, 1954), where eye and nose both are well developed. Hence, L. bata may be categorised as eye nose fish and this feature favours to its diurnal and benthopelagic habit. The median depression on the rosette due to radial arrangement of lamellae enhances water retention time as well as interaction time of odorant molecules with the receptors. | ||||||||||
| The sensory epithelium of this fish, L. bata is located on the lateral sides of the lamellae which correspond to the type-I (sensory epithelium continuous except in the margin) category under the classification according to Yamamoto (1982). The type-I distribution of sensory epithelium is considered as the highly capable olfactory organ among fish. As found in other teleosts (Yamamoto, 1982; Zielinski and Hara, 1988; Hara, 1993) the olfactory epithelium of L. bata also consists of at least two morphologically distinct types of ORCs, ciliated and microvillous among them ciliated ORC are dominant. The functional differentiation of the ciliated and microvillous receptor cells is still not clear. However, some reports have stated that the cORC responds to variety of odorants including amino acids, bile salts whereas, mORC is specialised to respond to amino acids and nucleotides (Hansen and Finger, 2000; Sato and Suzuki, 2001). Hence, presence of both these basic types of receptor cells in L. bata enables the fish to perceive most of the chemical signals. | ||||||||||
| The supporting cells surround the olfactory receptor cells in the sensory region provide ORC with mechanical support. Their apical surfaces consist of short cilia that help in holding and moving the mucous film over the epithelium. The supporting cells contain numerous mitochondria and secretion vesicles in their apical portion which indicate their function of secretion (Mokhtar and Abd-Elhafeez, 2014). In the present study, PAS positive reaction on the apical surfaces of supporting cells indicates their function of secretion. | ||||||||||
| The unique feature of olfactory epithelium is the presence of regenerative basal cells which are assumed to be the progenitor cells of the receptor and supporting cells (Zeiske et al., 1992). This study depicts the presence of elliptical and globular basal cells at the base of sensory epithelium. These cells replenish the lost cells during normal cell turn over or in case of epithelial injury. The increased mitotic figures and proliferation of the basal cells have been reported in case of epithelial injury (Evans et al., 1982; Roy et al., 2013). The non-sensory epithelium provides with the mechanical support to the sensory epithelium. The median raphe and the margins of lamellae contain stratified epithelial cells bearing microridges. These micoridges help in holding mucous film over the epithelium, and thus helps the epithelium in protection from mechanical abrasion. Mucous cells are distributed all over the epithelium and secrete both acid and neutral mucin and their secretion create a suitable medium for diffusion of odorants and help in smooth flow of water. The mucous film traps xenobiotics especially heavy metals (Roy et al., 2012) and removes or delays their penetration to the underlined olfactory tissue. The non-sensory epithelium also consists of ciliated cells. The beating of cilia of the ciliated non-sensory cells helps in ventilating the olfactory chamber. | ||||||||||
CONCLUSION` |
||||||||||
| A well-organized nasal ventilation system, large epithelium surface and presence of both basic types of olfactory receptor cells suggest that the olfactory organ of Labeo bata is well organized and seems to be efficient in perceiving chemical signals. | ||||||||||
ACKNOWLEDGMENTS |
||||||||||
| The authors are grateful to the Head of the Department of Zoology, Visva-Bharati University, Santiniketan for providing necessary laboratory facilities and to the authority of Centre for Research in Nano-Science and Nanotechnology (CRNN), University of Calcutta for extending help in using Scanning Electron Microscope. Thanks are due to the University Grants Commission, New Delhi for financial assistance in the form of Rajiv Gandhi National Fellowship to Ishita Samajdar. | ||||||||||
Figures at a glance |
||||||||||
|
||||||||||
References
|