Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2019) Volume 9, Issue 68
Evaluation of Neuroprotective Property of Madhunashini Vati in Diabetic Rat
Atiksha Rawat1*, Arun Kumar2, Preeti Kothiyal31Department of Pharmaceutical Sciences, UIPS, Uttaranchal University, Dehradun, India
2Department of Pharmaceutical Sciences, Himgiri Zee University, Dehradun, India
3Department of Pharmaceutical Sciences, SGRR University, Dehradun, India
- Corresponding Author:
- Atiksha Rawat
Department of Pharmaceutical
Sciences Uttaranchal University Dehradun, India
E-mail: atiksha.rawat91@gmail.com
Accepted date: August 02, 2019
DOI: 10.35841/2249-622X.67.19-257
Visit for more related articles at Asian Journal of Biomedical and Pharmaceutical SciencesAbstract
The present study was designed to evaluate the Neuroprotection property of Madhunashini Vati in Diabetic Rat. Eight groups of rat and each group comprising of six animals were employed in the present study. During the experiment different doses of Madhunashini Vati (125, 250, 500 mg/kg) was administered. Insulin (4 IU/kg body weight s.c) was administered daily before the global ischemia in diabetic rats. Rats were treated with Streptozotocin (STZ) 65 mg/kg i.p for the induction of Diabetes for 14 days. Rats were anaesthetized by using chloral hydrate (400 mg/kg, i.p) and Global cerebral ischemia was induced by occluding the carotid arteries, reperfusion was allowed for 24 hours. At the end of study, total Serum Glucose level, Serum Triglyceride level, Thiobarbituric Acid Reactive Substances(TBARS) and Cerebral Infarct size was estimated for the Neuroprotection property. Administration of Madhunashini Vati, showed reduction in the serum glucose level, serum triglycerides level, cerebral infarct size and increases in body weight. In the present study we observed that the madhunashini vati significantly improved cerebral ischemia. Results indicate that Madhunashini Vati (MV) is a good neuroprotective and could be used as a therapeutic treatment in brain stroke. And it is likely to be responsible for the free radical scavenging activity and rejuvenate the beta cells observed.
Keywords
Madhunashini Vati (MV), Streptozotocin (STZ), Neuroprotection, Thiobarbituric Acid Reactive Substances(TBARS).
Introduction
Stroke
Stroke is a long term complication of diabetes [1]. A patient with Diabetes has higher risk of stroke as compared to non-Diabetes. Diabetes and stroke both affects the blood vessels and both are associated with other risk factors such as hypertension, obesity, arterial fibrillation and dyslipidemia [2]. Different types of molecular signaling mechanism activated by hyperglycemia are Increased polyol pathway flux, Protein kinase C activation, Increased hexosamine pathway flux and Increased Advanced glycation end products (AGEs) formation [3] have demonstrated that elevation of extracellular glucose level leads to increased activity of Protein kinase C (PKC) and the mechanism of the activation is due to an enhanced denovo synthesis of diacylglycerol (DAG). Protein kinase C has been associated with vascular alteration such as increase in permeability, contractility, cell growth and apoptosis, angiogenesis, adhesion of leukocytes and activation of cytokine caused by different PKC isoforms [4]. Hyperglycemia increases polyol pathway flux, forming sorbitol and NADH. NADH and glucose stimulate the formation of Diacyclglycerol which in turn activates protein kinase C. Advance glycation end products (AGEs) that are produced by the glycation of proteins which also stimulate PKC or cause reactive oxygen species (ROS) activation directly. PKC is also activated by the fatty acids and angiotensin II [5].
Drug profile: (Madhunashini Vati) Madhunashini Vati is a herbal drug formulation that are used for diabetes mellitus. It is a combination of medicinal plants such as namely Tinospora cordifolia, Momordica muricata, Trigonella foenum graecum, Curcuma longa, Caesalpinia bonducella, along with Shilajit. It has a highly antidiabetic activity with equal proportion of shilajit. It has free radical scavenging activity of rejuvenation of cells that initiates to multiply the beta cells in pancreas with improving their potential to secrete insulin. momordica charantia and Tinospora cordifolia presence reported to decrease hyperglycemia and long term treatment of shilajit enhance the pancreatotrophic action (increases the number of beta cells) that may result in secretion of a large quantity of insulin in response to hyperglycemia. Madhunashini formulation has proved to be effective in nephrotoxicity [6].
Madhunashini as an antidiabetic herbal formulation has evaluated for acute and chronic toxicity studies in mice and rats respectively. In mice, the dose of Madhunashini formulation (MD-19) was found to be nontoxic up to 4000 mg/kg. For oral route, in mice LD50 was found to be 7500 mg/kg and 4500 mg/kg i.p. In rats oral dose was found to be 1000 mg/kg for 30 days did not show any toxicity effect. It showed also a dose dependent cardiac stimulant activity with a significant positive chronotropic and inotropic activity [7]. Madhunashini Vati is an ayurvedic formulation which activates the pancreas and helps to secrete a potential and balanced quantity of insulin, through which extra glucose gets converted into glycogen [8]. Insulin renders diabetic rats resistant through arrested nitric oxide reaction with superoxide and form peroxynitrite in acute ischemic stroke. Insulin improved the superoxide production and microvascular functions. Insulin improved endothelial dysfunction in brain. It improves the bioavalability of NO in brain which causes the vasodialation [9]. Insulin treatment in diabetic animals, decrease in lipid peroxides and neurolipofuscin deposition and restored the calcium levels and the membrane fluidity also [10].
Materials and Methods
Procurement of Madhunashini Vati (Formulation)
The marketed preparation of Madhunashini Vati was procured from Local market patanjali (Dehradun).
Procurement of animals
Albino wistar rat of either sex weighing 80-100 g was procured in the animal house facility of Shri Guru Ram Rai Institute of Technology and Sciences, Patel Nagar, Dehradun. Animals was acclimatized in the animal house facility of the department and housed in polypropylene cages with husk bedding (renewed every 48 h) under 12:12 light dark cycle at 25˚C ± 50˚C. and will be fed with standard Commercial pellet and water ad libitum.
The Experimental protocol was approved by Institution Animal Ethics Committee (Registration No. M.PH/IAEC/01/2015/ EEC-02) and care of animals was as per guidelines of Committee for the purpose of Control and Supervision of Experiments on Animals (Regdind Number 264/CPCSEA).
Induction of Type-I Diabetes Mellitus with STZ (Streptozotocin)
A single dose of streptozotocin (65 mg/kg) prepared in citrate buffer (pH 4.5, 0.1 M) was intraperitonially administered into overnight fasted rat to induce diabetes in all group except control group. All the rats were allowed free access to water, pallet diet and maintained at room temperature in polypropylene cages. STZ treated rats were also fed glucose solution 10% for 12 hours to avoid hypoglycemia. Rats having serum glucose more than 200 mg/dl after 2 week of induction were considered as diabetic and selected for further study.
Induction of global cerebral ischemia
Rats were anaesthetized by using chloral hydrate (400 mg/kg, i.p). A midline ventral incision was made in the neck to expose the right and left common carotid arteries, which were isolated from surrounding tissue and vagus nerve. A cotton thread was passed below both the carotid arteries. Global cerebral ischemia was induced by occluding the carotid arteries. After 17 minutes of global cerebral ischemia, reperfusion was allowed for 24 hours. After 17 minutes, incision was sutured back in layers. The sutured area was cleaned with 70% ethanol and was sprayed with antiseptic dusting powder. The animals were shifted individually to their home cage and were allowed to recover overnight. The animals were kept on a heating pad during surgery to maintain the body temperature, to avoid the effect of temperature variations on the final results [11].
Experimental Protocol
Control and Diabetic rat were randomly selected and divided into eight groups and each group comprises 6 animals.
Group I (Normal Control group): Normal Animals.
Group II (Sham Control): Only surgical procedure was performed (Carotid artery was exposed for 17 minutes).
Group III (Diabetic Control group): Rats were treated with streptozotocin (65 mg/kg. i.p) for the induction of type-1 diabetes.
Group IV (Diabetes+stroke induced group): 17 minutes global ischemia was induced in diabetic rats followed by 24 hours reperfusion.
Group V (Standard drug treated group in diabetes+stroke induced): Insulin (4 IU/kg body weight s.c) was administered daily for seven days before the global ischemia in diabetic rats.
Group VI (Test group): Madhunashini Vati (125 mg/kg body weight p.o) was administered daily for seven days before the global ischemia in diabetic rats.
Group VII (Test group): Madhunashini Vati (250 mg/kg body weight p.o) was administered daily for seven days before the global ischemia in diabetic rat.
Group VIII (Test group): Madhunashini Vati (500 mg/kg body weight p.o) was administered daily for seven days before the global ischemia in diabetic rats.
Insulin (standard) and Madhunashini Vati (test drug) were administered for next seven days starting from 2nd week of STZ injection, before the global ischemia in diabetic rats.
Collection of blood sample
Blood from the experimental rat was withdrawn by retro orbital plexus techniques under mild anesthesia using heparinized capillary glass tubes. Serum was separated by centrifugation [12].
Preparation of post-mitochondrial supernatant
Animals were sacrificed by cervical dislocation and then the brains were removed and cooled 0.9% saline and brain was kept on ice and subsequently blotted on filter paper, then weighed and homogenized in cold phosphate buffer (0.1M, pH 7.4) by using homogenizer. The homogenate was centrifuged at 10,000 rpm for 15 min at 4̊C and post-mitochondrial supernatant was kept on ice until assayed and used for biochemical estimation weighed and homogenized in phosphate buffer (pH 7.4). The homogenate were then centrifuged at 10,000 rpm for 15 min at 40̊C and postmitochondrial supernatant was kept on ice until assayed and used for biochemical estimation [13].
Analysis of biochemical parameters
Estimation of Glucose level: Serum glucose level was estimated by glucose oxidase/peroxidase method by using commercially available enzymatic glucose oxidase-peroxidase method. The blank, standard and test sample was prepared according to the standard procedure as mentioned in the standard serum glucose estimation kit. The absorbance was measured against blank 540 nm using spectrophotometer [14].
Estimation of tissue TBARS level: it was estimated by using the method describes by Slater and Sawyer. 2.0 ml of the tissue homogenate called (supernatant) was added to 2.0 ml of freshly prepared 10% w/v trichloroacetic acid (TCA) and the mixture was allowed to stand in an ice bath for 15 minutes. After 15 minutes, the precipitate was separated through centrifugation and 2.0 ml of clear supernatant solution was mixed with 2.0 ml, freshly prepared of thiobarbituric acid (TBA). Then resulting solution was heated in a boiling water bath for mainly 10 minutes. Then it was immediately, cooled in an ice bath for 5 minutes. The developed color was measured at 532 nm [11,15].
Assessment of cerebral infarct size: At the end of 24 hours of reperfusion after global cerebral ischemia, the animals were sacrificed by cervical dislocation. Brain samples were immediately sliced and slices were incubated with 1% triphenyltetrazoliumchloride (TTC) at 37 ̊C in 0.2M Tris buffer (pH7.4) for 20 minutes. TTC is converted to red form zone pigment by NAD and lactate dehydrogenase and thus stained the viable cells deep red. The Infarcted cells lost the enzyme as well as cofactor and thus remain unstained dull yellow. The average area of each brain slice was calculated by counting the number of squares on either side. Similarly, the number of squares falling over non-stained dull yellow area was also counted. Infarcted area was expressed as a percentage of the total brain volume. Whole brain slices were weighed. Infarcted dull yellow part was dissected out and weighed. Infarct size was expressed as percentage of the total wet weight of the brain [11].
Estimation of serum triglyceride level: Triglyceride level was estimated by using commercially available triglycerides enzymatic assay kit. The absorbance of the colored complex is measured at 505 nm [16].
Statistical analysis
All the entire data were expressed as mean standard error mean (SEM). All the data was analyzed through one way ANOVA, followed by tukey’s test for significance except body weight and glucose level. Data of Body weight and glucose level was analyzed through two ways ANOVA, followed by Bonferroni’s multiple comparison test using graph pad prism version 5.3 software.
Results
Effect of Madhunashini Vati on body weight (g)
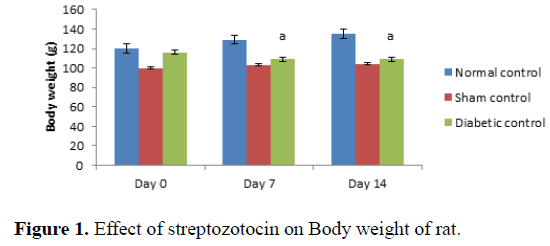
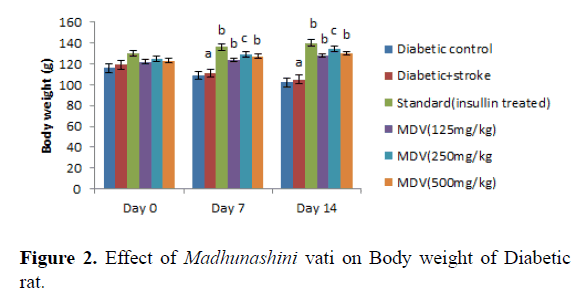
Administration of streptozotocin in rat produced significant decreases in body weight in rats as compared to normal control (Table 1 and Figure 1). Treatment with Madhunashini Vati in diabetic rats (125, 500 mg/kg p.o) showed significant (P ≤ 0.01) increase in body weight as compared to diabetic control group and 250 mg/kg p.o showed significant (P ≤ 0.05) increase in body weight as compared to diabetic+stroke group (Table 2 and Figure 2).
| Group | Day 0 | Day 7 | Day 14 |
|---|---|---|---|
| Normal control | 120 ± 0.991 | 129 ± 0.972 | 135 ± 1.202 |
| Sham control | 100 ± 0.970 | 103 ± 0.960 | 104 ± 0.988 |
| Diabetic control | 116 ± 1.321 | 109 ± 0.991a | 102 ± 1.232a |
Table 1. Effect of Streptozotocin on body weight in rat Body weight (g) mean ± SEM.
| Group | Day 0 | Day 7 | Day 14 |
|---|---|---|---|
| Diabetic Control | 116 ± 1.321 | 109 ± 0.991 | 102 ± 0.942 |
| Diabetic+Stroke | 119 ± 1.623 | 111 ± 1.132a | 105 ± 0.982a |
| Standard (Insulin) in diabetic rat | 130 ± 0.922 | 136 ± 1.423b | 140 ± 1.665b |
| MD(125 mg/kg)in diabetic rat | 122 ± 1.510 | 124 ± 1.399b | 128 ± 1.021b |
| MD(250 mg/kg)in diabetic rat | 125 ± 1.151 | 129 ± 1.344c | 134 ± 1.211c |
| MD(500 mg/kg)in diabetic rat | 123 ± 0.933 | 127 ±0.972b | 130 ± 0.912b |
Table 2. Effect of Madhunashini Vati on body weight in Diabetic rat Body weight (g) mean ± SEM.
Day 0 represents, no STZ was administered. Same day, STZ was administered and body weight was measured on 0, 7th and 14th day. Values are expressed as mean ± SEM, n=6, a represents P ≤ 0.01: As compared to normal control group.
Control group represents, there was no STZ administered. Sham control represent as, there was surgical procedure was performed. Administration of STZ produced a significant decrease in body weight of rats as compared to normal control. Values are expressed as mean ± SEM, n=6, a represents P ≤ 0.01: As compared to normal control group.
Day 0 represents, no STZ was administered. Same day, STZ was administered and after administered of STZ, on day 0, 7th and 14th day body weight was measured. Values are expressed as mean ± SEM, n=6, a represents P ≤ 0.001: As compared to Diabetic control group. B represents P ≤ 0.01 As compared to Diabetic+stroke group. C represents P ≤ 0.05 : As compared to diabetic+stroke control.
Control group represents, there was no STZ administered. Sham control represent as, there was surgical procedure was performed. Administration of STZ produced a significant decrease in body weight of rats as compared to normal control. Administration of Madhunashini Vati in diabetic rat (125 mg/kg, 250 mg/kg, and 500 mg/kg p.o) produced significant (P˂ 0.001) increases in body weight as compared to diabetic control group. Values are expressed as mean ± SEM, n=6, A represents P ≤ 0.001: As compared to Diabetic control group. B represents P ≤ 0.01: As compared to Diabetic control. C represents P ≤ 0.05: As compared to diabetic+stroke group.
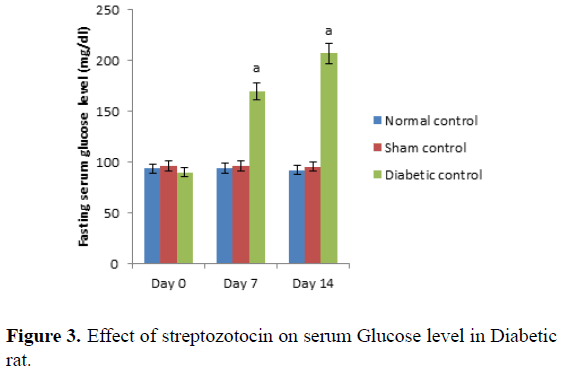
Day 0 represents, no STZ was administered. Same day, STZ was administered and after administered of STZ, on day 0, 7th and 14th day serum glucose level was measured. Values are expressed as mean ± SEM, (n=6). ‘A’ represents P ≤ 0.001 : As compared to normal control group (Table 3 and Figure 3).
| Group | Day 0 | Day 7 | Day 14 |
|---|---|---|---|
| (After 14 days STZ administration) | (After 21 days STZ administration) | (After 28 days STZ administration) | |
| Normal control | 93.55 ± 0.95 | 94.23 ± 0.99 | 92.22 ± 0.94 |
| Sham control | 96.65 ± 0.98 | 95.98 ± 1.01 | 95.65 ± 0.90 |
| Diabetic control | 90.42 ± 2.11 | 170.15 ± 1.72a | 207.32 ± 0.91a |
Table 3. Effect of Streptozotocin on Serum Glucose level in Diabetic rat Fasting Serum Glucose level (mg/dl) mean ± SEM.
Control group represents, there was no STZ administered. Administration of STZ produced significant (P ≤ 0.001) increases in fasting serum glucose level in rats as compared to normal control. Values are expressed as mean ± SEM, (n=6). ‘A’ represents P ≤ 0.001 : As compared to normal control group (Table 4 and Figure 4).
| Group | Day 0 | Day 7 | Day 14 |
|---|---|---|---|
| (After 14 days STZ administration) | (After 21 days STZ administration) | (After 28 days STZ administration) | |
| Diabetic control | 207.42 ± 2.11 | 220.15 ± 1.72a | 249.32 ± 0.91a |
| Diabetic+stroke | 200.07 ± 1.87 | 217.53 ± 1.98 | 243.34 ± 1.28 |
| Standard(Insulin) in diabetic rat | 202.35 ± 2.21 | 197.21 ± 1.53b | 185.35 ± 1.65b |
| MDV(125 mg/kg) in diabetic rat | 205.25 ± 2.25 | 194.46 ± 2.87b | 182.24 ± 1.92b |
| MDV(250 mg/kg) in diabetic rat | 201.33 ± 2.28 | 192.23 ± 1.99b | 186.42 ± 1.20b |
| MDV(500 mg/kg) in diabetic rat | 202.46 ± 2.21 | 199.32 ± 1.56b | 187.45 ± 1.12b |
Table 4. Effect of Madhunashini Vati on Serum Glucose level in Diabetic rat Fasting Serum Glucose level (mg/dl) mean ± SEM.
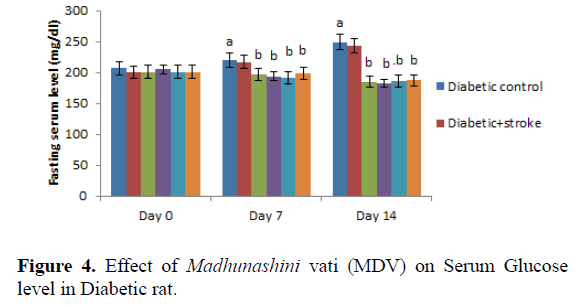
Day 0 represents,administration of Insulin and MDV (Madhunashini Vati) in diabetic rat and after 14th day serum glucose level was measured. Values are expressed as mean ± SEM, (n=6). ‘A’ represents P ≤ 0.001 : As compared to control group. B represents P ≤ 0.001 : As compared to normal control group.
Administration of MDV in diabetic rat (125 mg/kg, 250 mg/kg, and 500 mg/kg p.o) treatment did not produced a significant effect on serum glucose level in diabetic rats on day 0. Then, administration of MDV treatment in diabetic rats on day 7th and 14th showed a significant (P˂0.001) lowering the serum glucose level as compared with diabetic control. Values are expressed as mean ± SEM, (n=6). A represents P ≤ 0.001: As compared to control group. B represents P ≤ 0.001: As compared to Diabetic control (Table 5 and Figure 5).
| Group | Triglycerides (mg/dl) |
|---|---|
| Normal control | 114.01 ± 2.01 |
| Sham control | 112.13 ± 2.12 |
| Diabetic control | 185.25 ± 2.52a |
| Diabetic+Stroke | 195.57 ± 2.62a |
| Standard (Insulin treated) | 165.32 ± 2.52b |
| MD (125 mg/kg) | 152.37 ± 2.35b |
| MD(250 mg/kg) | 148.44 ± 2.66b |
| MD(500 mg/kg) | 141.22 ± 2.88b |
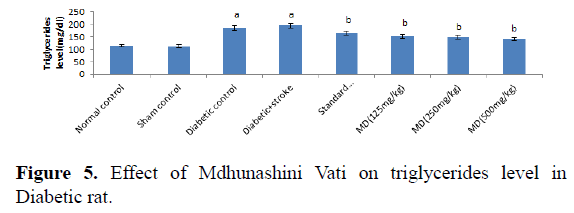
Table 5. Effect of Madhunashini Vati on triglycerides level in Diabetic rat.
Control group represents, brain was removed without performed surgical procedure. Sham group represent, the surgical procedure was performed in each rat, both carotid arteries were isolated and exposed for 17 minutes followed 24 hours reperfusion and brain was removed to estimate the triglycerides level. Administration of MDV Madhunashini Vati (125 mg/kg, 250 mg/kg, and 500 mg/kg p.o) in diabetic rat and after 7 days triglycerides level was measured. Values are expressed as mean ± SEM (n=6). A represents P ≤ 0.001: As compared to normal control group. B represents P ≤ 0.001: As compared to diabetic control group.
Administration of STZ in rat produced a significant increase in serum triglycerides level in rats as compared to normal control. Administration of MDV (125 mg/kg, 250 mg/kg, and 500 mg/kg p.o) in diabetic’s rats showed a significant (P≤0.001) lowering of serum triglycerides level after 7 days as compared with diabetic control. Values are expressed as mean ± SEM (n=6). A represents P ≤ 0.001: As compared to normal control group. B represents P ≤ 0.001: As compared to diabetic control group (Table 6 and Figure 6).
| Group | Cerebral infarct size (by weight %) |
|---|---|
| Normal control | 2.45 ± 0.92 |
| Sham control | 2.52 ± 0.95 |
| Diabetic control | 32.21 ± 2.54a |
| Diabetic+Stroke | 44.45 ± 2.82a |
| Standard (Insulin treated) | 34.91 ± 1.22c |
| MD (125 mg/kg) | 40.75 ± 1.12b |
| MD(250 mg/kg) | 36.42 ± 1.09b |
| MD(500 mg/kg) | 35.45 ± 2.12b |
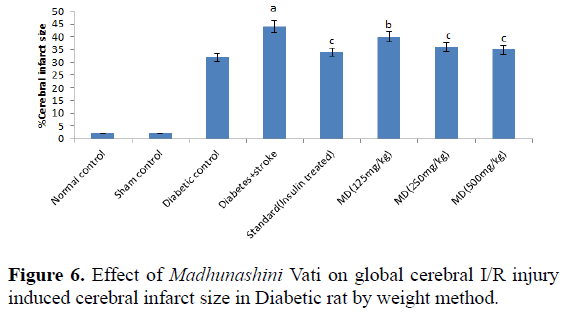
Table 6. Effect of Madhunashini Vati on global cerebral I/R injury induced cerebral infarct size in Diabetic rat by weight method.
Control group represents, brain was removed without performed surgical procedure. Sham group represent, the surgical procedure was performed in each rat, both carotid arteries were isolated and exposed for 17 minutes followed 24 hours reperfusion and brain was removed to estimate the cerebral infarct size by weight method. Administration of MDV Madhunashini Vati (125 mg/kg, 250 mg/kg, and 500 mg/kg p.o) in diabetic rat and after 7 days cerebral infarct size was measured by weight method. Values are expressed as mean ± SEM (n=6). A represents P ≤ 0.01: As compared to normal control group. B represents P ≤ 0.001: As compared to diabetic +stroke control group. C represents P ≤ 0.05: As compared to diabetes+stroke group.
Global cerebral ischemia of 17 minutes followed by 24 hours reperfusion produced a significant increase in cerebral size in Diabetic+stroke group as compared to normal control group and diabetic group. There is no significant differences in control and sham group by both weight and volume methods. After 7 days prior to treatment of Madhunashini Vati (125 mg/kg, 250 mg/kg, 500 mg/kg p.o) in diabetic rats significantly (P˂0.001, P˂0.01) decreases the cerebral infarct size as compared to Diabetes+stroke group. Values are expressed as mean ± SEM (n=6). A represents P ≤ 0.01: As compared to normal control group. B represents P ≤ 0.001: As compared to diabetic+stroke group. C represents P ≤ 0.05: As compared to diabetes+stroke group (Table 7 and Figure 7).
| Group | Cerebral infarct size (by volume %) |
|---|---|
| Normal control | 2.25 ± 0.94 |
| Sham control | 2.45 ± 0.97 |
| Diabetic control | 35.48 ± 1.95 |
| Diabetic+Stroke | 46.55 ± 2.05a |
| Standard (Insulin treated) | 36.11 ± 0.98c |
| MD (125 mg/kg) | 42.05 ± 1.05b |
| MD (250 mg/kg) | 38.05 ± 2.21b |
| MD (500 mg/kg) | 35.34 ± 2.16b |
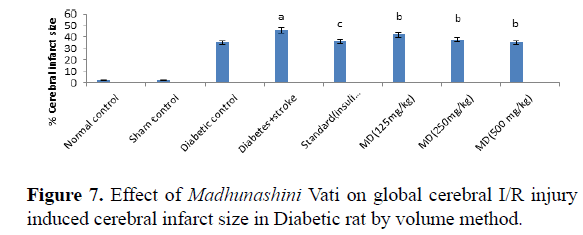
Table 7. Effect of Madhunashini Vati on global cerebral I/R injury induced cerebral infarct size in Diabetic rat by volume method.
Control group, brain was removed without performed surgical procedure. Sham group represent, the surgical procedure was performed in each rat, both carotid arteries were isolated and exposed for 17 minutes followed 24 hours reperfusion and brain was removed to estimate the cerebral infarct size by volume method. Administration of MDV Madhunashini Vati (125 mg/kg, 250 mg/kg, and 500 mg/kg p.o) in diabetic rat and after 7 days cerebral infarct size was measured by volume method. Values are expressed as mean ± SEM (n=6). A represents P ≤ 0.001: As compared to normal control group. B represents P ≤ 0.001: As compared to diabetic+stroke group. C represents P ≤ 0.05: As compared to diabetes+stroke group.
Global cerebral ischemia of 17 minutes followed by 24 hours reperfusion produced a significant increase in cerebral size in Diabetic+stroke group as compared to normal control group and diabetic group. There is no significant differences in control and sham group by both weight and volume methods. After 7 days prior to treatment of Madhunashini Vati (125 mg/kg, 250 mg/kg, 500 mg/kg p.o) in diabetic rats significantly (P ≤ 0.001, P ≤ 0.01) decreases the cerebral infarct size as compared to Diabetes+stroke group. Values are expressed as mean ± SEM (n=6). A represents P ≤ 0.001: As compared to normal control group. B represents P ≤ 0.001: As compared to diabetic+stroke group. C represents P ≤ 0.05: As compared to diabetes+stroke group.
Control group, brain was removed without performed surgical procedure. Sham group represent, the surgical procedure was performed in each rat, both carotid arteries were isolated and exposed for 17 minutes followed 24 hours reperfusion and brain was removed to estimate the TBARS level in diabetic rats. Administration of MDV Madhunashini Vati (125 mg/kg, 250 mg/kg, and 500 mg/kg p.o) in diabetic rat and after 7 days TBARs level was measured. Values are expressed as mean ± SEM, (n=6). A represents P ≤ 0.001: As compared to normal control group. B represents P ≤ 0.001: As compared to diabetic control group. C represents P ≤ 0.001: As compared to diabetes +stroke group (Table 8).
| Group | TBAR level (nmol/mg protein) |
|---|---|
| Normal control | 2.82 ± 0.94 |
| Sham control | 2.86 ± 0.92 |
| Diabetic control | 4.12 ± 1.23a |
| Diabetic+Stroke | 8.35 ± 1.21b |
| Standard (Insulin treated) | 5.18 ± 0.99c |
| MD (125 mg/kg) | 4.07 ± 0.99c |
| MD (250 mg/kg) | 5.38 ± 0.94c |
| MD (500 mg/kg) | 3.58 ± 1.23c |
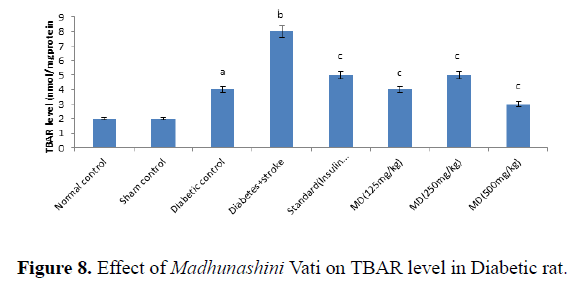
Table 8. Effect of Madhunashini Vati on Increases in Thiobarbituric acid reactive substances (TBARS) level in Diabetic rat.
Global cerebral ischemia of 17 minutes followed by 24 hours reperfusion produced a significant increase in TBARS level in Diabetic and diabetic+stroke group as compared to normal control group. There is no significant differences in control and sham group by both weight and volume methods. After 7 days prior to treatment of Madhunashini Vati (125 mg/kg, 250 mg/kg, 500 mg/kg p.o) in diabetic rats significantly (P ≤ 0.001, P ≤ 0.01) decreases the TBARS level as compared to Diabetes +stroke group. Values are expressed as mean ± SEM, (n=6). A represents P˂0.001: As compared to normal control group. B represents P˂0.001: As compared to diabetic control group. C represents P˂0.001: As compared to diabetes+stroke group (Figure 8).
Discussion
In the present study, to evaluate the neuroprotective effect of Madhunashini Vati in streptozotocin induced and global cerebral ischemia/reperfusion injury induced in rat. It is well known as that diabetes has higher risk of stroke. Diabetes and stroke both affects the blood vessels and both are associated with other risk factors such as: hypertension, obesity, arterial fibrillation and dyslipidemia [2]. Overproduction of ROS by mitochondria causes the impairment of ETC (electron transport chain), which leads to decreased ATP production and increased the formation of free radicals, altered calcium homeostasis and mitochondrial dysfunction [17]. Many synthetic drugs can protect against oxidative damage, but they have side effects also. Numerous clinical and preclinical studies using especially tissue plasminogen activator thrombolytics agents examined pharmacological recanalization and restoration of cerebral circulation or reperfusion within minutes of the onset of treatment. It is also recently was shown to be effective and approved for the urgent treatment of ischemic stroke in humans. But the less effective, widely applicable and safe pharmacological treatments of ischemic patients that may grow interest towards the traditional medicines. It has been suggested that in brain, herbal medicines or their products may improve the microcirculation, protect ischemic reperfusion injury also and possess neuroprotective properties that leads to inhibit apoptosis. So there is an alternative solution for this problem is to consume natural antioxidants as much as possible from food supplements and traditional medicines [18].
In this study Madhunashini Vati was used as a test drug in rats. As per the previous reports that Madhunashini Vati combination herbal formulation namely Tinospora cordifolia, Momordica muricata, Trigonella foenum graecum, Curcuma longa, Caesalpinia bonducella, along with Shilajit and has an anti-diabetic activity due to the presence of shilajit. It has free radical scavenging activity of rejuvenation of cells that initiates to multiply the beta cells in pancreas with improving their potential to secrete insulin. momordica charantia and Tinospora cordifolia presence reported to decrease hyperglycemia and long term treatment of shilajit enhance the pancreatotrophic action (increases the number of beta cells) that may result in secretion of a large quantity of insulin in response to hyperglycemia [6]. And previous study also investigated that, Trigonella foenum graecum seeds one of the ingredients of Madhunashini Vati has neuroprotective property. It has shown the presence of steroid saponins in TSP Trigonella foenum graecum seeds, which rejuvenate the pancreas beta cells and stimulate the insulin secreation [10]. Diabetes mellitus (Type 1) was chemically induced in rat by administrating single dose of streptozotocin (STZ 65 mg/kg i.p) which produces cytotoxicity to beta cells of islets of langerhans by increasing the activity of xanthine oxidase and poly ADP- ribose polymerase (PARP), that causes apoptotic and necrotic cell death in beta cells of pancreas. In this study, Anti-diabetic effects of Madhunashini Vati were evaluated because diabetes is the leading cause of brain damage due to several mechanisms.
Madhunashini Vati, showed reduction in the serum glucose level, serum triglycerides level, MDA (malondialdehyde), cerebral infarct size and increases in body weight as compared to diabetic control and diabetes+stroke group. Low dose shows less effect as compared to medium dose and high dose. But medium dose of Madhunashini Vati (250 mg/kg p.o) in diabetic rat showed significant increases in body weight as compared with low and high dose. Madhunashini Vati (125 mg/kg, 250 mg/kg, 500 mg/kg p.o) did not showed any significant effect on body weight on day 0 but it showed a significant increases in body weight on 7th and 14th day. Madhunashini Vati (125 mg/kg, 250 mg/kg, and 500 mg/kg p.o) in diabetic rat showed a significant decrease in serum glucose level as compared with diabetic group. On 14th day, Madhunashini Vati (125 mg/kg p.o) showed a significant decrease in serum glucose level as compared with medium and high dose of madhunshini vati in diabetic rat. In the present study we observed that the Madhunashini Vati significantly improved cerebral ischemia. Diabetic control group showed a significant difference as compared to normal control and sham control group when the increased level of markers of oxidative stress (brain damage) was observed and this finding of the present study suggest the diabetes mellitus play a key role in the pathogenesis of oxidative stress and brain stroke. Results indicate that Madhunashini Vati is a good neuroprotective and could be used as a therapeutic treatment in brain stroke. And it is likely to be responsible for the free radical scavenging activity and rejuvenate the beta cells observed.
Conclusions
From the above discussion and results it can be concluded that:
Madhunashini Vati shows a Neuroprotective as well as anti-diabetic effect in streptozotocin induced diabetic rat. Treatment with Madhunashini Vati (125 mg/kg, p.o) shows to lower serum glucose level due to may be increased glucose uptake by the tissues and its utilization and it also increases the body weight, also it has good scavenger of free radicals.
There was a significant decrease in cerebral infarct size and triglycerides level at the dose of 500 mg/kg p.o of Madhunashini Vati, which are the main markers of brain damage.
The present study results constitute the main evidence for the therapeutic potential of Madhunashini Vati in Global Cerebral Ischemia/ Reperfusion Injury. The study supports an important concept that the onset of neurodegeneratives due to diabetes may be delayed or mitigated with use of anti-diabetic drugs with free radicals scavenger that protect against the brain damage, oxidative stress and degeneration of neurons.
Madunashini vati was found to be effective as neuroprotective because it might have scavenging activity of free radicals and rejuvenate the beta cells that initiates to multiply the beta cells in pancreas with improving their potential to secrete insulin which act as neuroprotection.
Acknowledgment
Authors would like to Thank Dr Preeti Kothiyal and Dr. Arun Kumar for their guidance and moral support during research work.
References
- Boden-Albala B, Sacco R. Lifestyle Factors and Stroke Risk: Exercise, Alcohol, Diet, Obesity, Smoking, Drug Use And Stress, Current Atherosclerosis Factors. 2000; 2: 60-166.
- Patel CA, Zhang J. Cardiovascular complications of diabetes mellitus: J Applied Pharmaceut Sci. 2011; 1: 1-6.
- Geraldes P1, King GL. Activation of PKC isoforms and its impact on diabetic complication: Circ Res. 2010;106: 1319-31.
- Gutterman DD Vascular dysfunction in hyperglycemia: is protein kinase C the culprit Circ Res. 2002;990:pp 5-7.
- Tiwari BM, Kumar D, Abidi AB. Antidiabetic activity of madhunshini (MD-19) in alloxan induced dibetes mellitus. J of Cell Tissue Res. 2011; 111: 2515-2520.
- Burade KB, Naikwade N.S, Chopade A.R, et al.. Acute Chronic Toxicity studies of an Anti-diabetic Formulation Madhunashini. J Herbal Med Toxicol. 2009; 3: 133-138.
- Hung LM, Huang JP, Liao JM. Insulin renders diabetic rats resistant to acute ischemic stroke by arresting NO reaction with superoxide to form peroxynitric. J Biomedi Sci. 2014; 21:92 .
- Kumar P, Kale RK, McLean P, Baquer NZ. Antidiabetic and Neuroprotection Effects of Trigonella Foenum-graecum Seed Powder in Diabetic Rat Brain. Prague Medical Report. 2012; 113: 33-43.
- Dhyani PM, Kumar P, Kothiyal P et al. Neuroprotective activity of Ascophyllum Nodosum on experimentally induced global cerebral ischemia/reperfusion injury in mice. Int J Pharmaco. 2013; 12: 53-160.
- Morani AS, Muthal AP, Mohan V, et al. Standardized extract of rigonella foenum- graecum L. seeds attenuates neuropathic pain in diabetic rats. Int Res J Pharmaceut Biosci. 2015; 23: 22-33.
- Sharma V, Subrahmanya GS, Kumar AG. The Effect of Minocycline on Oxidative stress and Memory Deficits in Aged Rats. Int J Pharmacy. 2012; 21: 71-79.
- Trinder P. Glucose Oxidase method. Ann Clin Biochem. 1969; 6: 22-29.
- Slater T.F, sawyer B.C. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. Biochem J. 1971; 123: 805-814.
- Rong Chen, MD, MS, Bruce Ovbiagele, MD, and Wuwei Feng, MD, MS. Diabetes: a risk factor for mortality from brain stroke. Indian J Pharmaceu Bio Research. 2015; 352: 28-36.
- Andreyev AY1, Kushnareva YE, Starkov AAMitochondrial metabolism of reactive oxygen species. Biochem. 2015; 70: 200-214.
- Jaiswal BS, Tailang M. Cerebral ischemic-reperfusion injury & Neuroprotective activity of herbal drugs. World J Pharmaceut Res. 2015; 4: 1653-1667.
- A. Akbarzadeh,corresponding author1 D. Norouzian,1 M. R. Mehrabi et al., Induction of diabetes by streptozotocin in rats”. Indian J Clinical Biochem. 2015; 222: 60-64.
- Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clinical Neurology and Neurosurgery 2009; 1116: 483-495.