- International Journal of Pure and Applied Zoology (2016) Volume 4, Issue 2
Evaluation of Cadmium (Cd) in Domestic Chicken Meat and Offal and Associated Health Risk Assessment in Ibadan
- *Corresponding Author:
- Emmanuel Teryila Tyokumbur
Department of Zoology
University of Ibadan, Ibadan, Oyo Nigeria, Nigeria
E-mail: e.tyokumbur@mail.ui.edu.ng
Received 11th March 2016; Accepted 18th April 2016; Published 28th April 2016
Abstract
The study was carried out on the evaluation of cadmium in chicken meat and offal in three markets in Ibadan. The appraisal was aimed at assessing the cadmium that has accumulated in the kidney, liver, intestine, blood, muscle and feathers of chicken widely consumed in domestic chicken (Gallus gallus domesticus). Ten mature chickens were bought and dissected to remove the chicken meat and offal samples. This samples were oven-dried separately and pulverised into powdered form through grinding with a mortar and pestle. These were acid-digested for cadmium analyses using Buck Scientific 210 VGP Atomic Absorption Spectrophotometer. The overall results from all the study sites showed varying mean concentrations in the intestine (0.713 ± 0.024 ppm), feather (0.702 ± 0.035 ppm) and muscle (0.592 ± 0.019 ppm) with low mean concentrations in the blood (0.426 ± 0.032 ppm), liver (0.432 ± 0.021 ppm) and kidney (0.352 ± 0.027 ppm). Most of the chicken meat and offal samples were above the WHO guideline limit of 0.05 ppm. The Target Hazard Quotient (THQ) from the study was below 1 which suggested that the general populace in this study area was not at risk.
Keywords
Chicken meat, Offal, Cadmium, Food composition, Bioaccumulation
Introduction
All mineral elements have the potential to cause toxicosis in higher consumers, especially when consumed in fairly significant quantities as part of food (Pond et al., 1995; ASTDR, 2005). The margin of safety between the minimum amounts required to feed on and the amount that causes adverse effects varies for different minerals, depending on prevailing conditions. However, there are many minerals that do not have any known function in the animal body and are therefore harmful or toxic. Today, environment, plants, animals and humans are exposed to high levels of these toxic minerals, and even higher than ever recorded in the past due to anthropogenic activities. This is attributable to industrial activities and the limitless burning of coal, gas and oil and incineration of waste materials that takes place around the world (Nriagu 1980; 1988; 1989; Nriagu and Pacyna 1988). Thus, the toxic heavy metals are ubiquitous thereby having the potential to bioaccumulate and transferred along the food chain to higher consumers like man. Among existing toxic heavy metals, the most important are arsenic, cadmium, lead and mercury, which are harmful to animals? health (Suttle 2010). The high influx of electronic wastes (e-wastes) rich in cadmium in Nigeria has the potential of finding its way into the food chain through bioaccumulation. Hence there is the need to assess cadmium levels in the chicken meat and offal consumed in Ibadan city, Nigeria. The aim of this study is to evaluate organ and tissue concentration of cadmium in domestic chicken as an indicator on the composition of tissues and its consequences when consumed by man. Thus, the main objectives of this study were to evaluate the bioavailability of cadmium in chicken meat and offal in Ibadan; assess the differences in tissues and organ levels of cadmium from different markets in Ibadan; compare the mean cadmium concentration in chicken meat and offal with World Health Organisation (WHO) and Food and Agricultural Organisation (FAO) guideline standards.
Materials and Methods
Study sites
The study sites for the collection of domestic chicken samples were the markets of Bodija (Ibadan North Local Government Area), Ojoo (Akinyele Local Government Area) and Sango in Ibadan city of Oyo State in Nigeria.
Sample collection, processing and analyses
A total of ten domestic chickens were randomly bought from Bodija (tagged as samples A, B, H, I, J), Ojoo (tagged as samples C, D) and Sango (tagged as samples E, F, G) markets. The domestic chickens bought were of market size and tagged A-J. Each chicken was slaughtered and dissected to remove blood, feather, liver, intestine, muscle and kidney all amounting to a total sample size of 60. The blood sample was allowed to coagulate naturally and together with other organs and tissues separately dried in an oven at 220°C for one hour. Each dry sample was pulverised using a pestle and mortar. 0.5-1 g of each powdered sample was digested overnight in 2 mL of 70% HNO3. Thereafter, 1 mL of 30 H2O2 was added and allowed to simmer down. Distilled water was added to the 10 mL mark of the test tube and taken to the laboratory for analyses. The digested and processed sample was analysed for cadmium (Cd) using Buck Scientific 210 VGP Atomic Absorption Spectrophotometer in triplicates. Standards and blanks were used for quality assurance of the results for each of the sixty samples.
Data analyses
The mean and standard deviation of the cadmium concentration in the chicken meat and offal from the study will be computed using Microsoft Excel. Maximum and minimum values of cadmium concentration in the chicken meat and offal will also be computed. The data showing variations in cadmium concentration in the chicken meat and offal at the three sampling sites will be plotted as histograms. This will show organ/tissue-based and spatial variations in comparison with Food and Agricultural Organisation/World Health Organisation (FAO/WHO) guidelines standards.
Health risk assessmentThe health risk assessment of the cadmium in the edible chicken parts would be carried out using the Target Hazard Quotient (THQ) equation. The methodology for estimation of target hazard quotient offers an indication of the risk level due to pollutant exposure (Cd) and is available in USEPA Region 111 risk-based concentration table (USEPA 2010). If THQ value is equal to or greater than 1, an exposed population to the pollutant of concern (Cd) will be at health risk.
 (1)
(1)
Where,
EF is exposure frequency (from 365 days per year for people who eat chicken meat 7 times a week to 52 days per year for people who eat chicken meat once a week).
ED is the exposure duration (70 years) equivalent to the average lifetime.
FIR is the food ingestion rate (for egg: 37.2 g/person/day) and used for chicken meat and offal.
C is the metal concentration in the chicken meat and offal (ppm).
RFD is the oral reference dose (USEPA 1997, 2000).
WAB is the average body weight (64 kg, the reference weight for the age categories)
TA is the average exposure time for non-carcinogens (365 day/year * ED).
Results
Cadmium level in the chicken meat and offal in Ibadan
Table 1 shows the mean cadmium levels, standard deviation, minimum and maximum values in the chicken meat and offal in Ibadan. The highest mean cadmium level from Bodija market was in the feather (0.068 ± 0.030), while the lowest was in the kidney (0.029 ± 0.033). The highest mean cadmium level from Sango market was in the intestine (0.086 ± 0.007), while the lowest was in the blood (0.037 ± 0.013). The highest mean cadmium level from Ojoo market was in the feather (0.117 ± 0.011), while the lowest was in the blood (0.017 ± 0.020).
| Market | Measure | Blood | Feather | Intestine | Blood | Feather | Intestine |
|---|---|---|---|---|---|---|---|
| BODIJA | Mean | 0.056 | 0.068 | 0.059 | 0.056 | 0.068 | 0.059 |
| Standard deviation | ± 0.039 | ± 0.030 | ± 0.029 | ± 0.039 | ± 0.030 | ± 0.029 | |
| Min-Max | 0.013-0.094 | 0.033-0.106 | 0.028-0.095 | 0.013-0.094 | 0.033-0.106 | 0.028-0.095 | |
| SANGO | Mean | 0.037 | 0.043 | 0.086 | 0.037 | 0.043 | 0.086 |
| Standard deviation | ± 0.013 | ± 0.018 | ± 0.007 | ± 0.013 | ± 0.018 | ± 0.007 | |
| Min-Max | 0.027-0.052 | 0.032-0.064 | 0.078-0.090 | 0.027-0.052 | 0.032-0.064 | 0.078-0.090 | |
| OJOO | Mean | 0.017 | 0.117 | 0.081 | 0.017 | 0.117 | 0.081 |
| Standard deviation | ± 0.020 | ± 0.011 | ± 0.010 | ± 0.020 | ± 0.011 | ± 0.010 | |
| Min-Max | 0.003-0.031 | 0.109-0.124 | 0.074-0.088 | 0.003-0.031 | 0.109-0.124 | 0.074-0.088 |
Table 1: Mean cadmium levels, standard deviation, minimum and maximum values in the chicken meat and offal in Ibadan
Variation of cadmium levels in chicken at the sampling sites
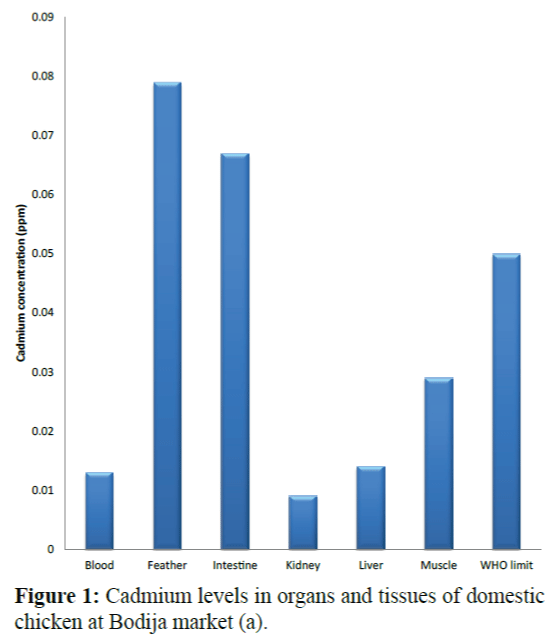
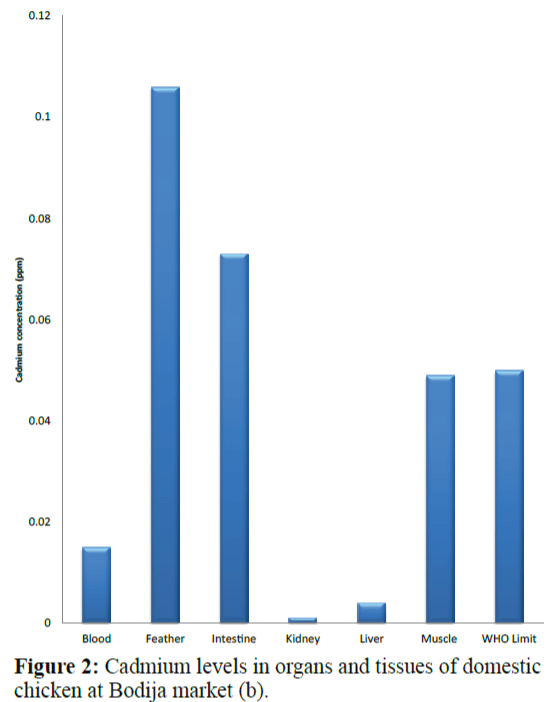
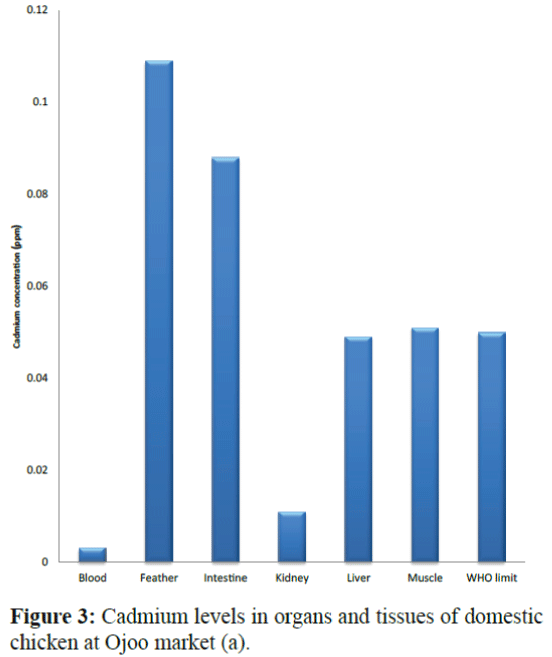
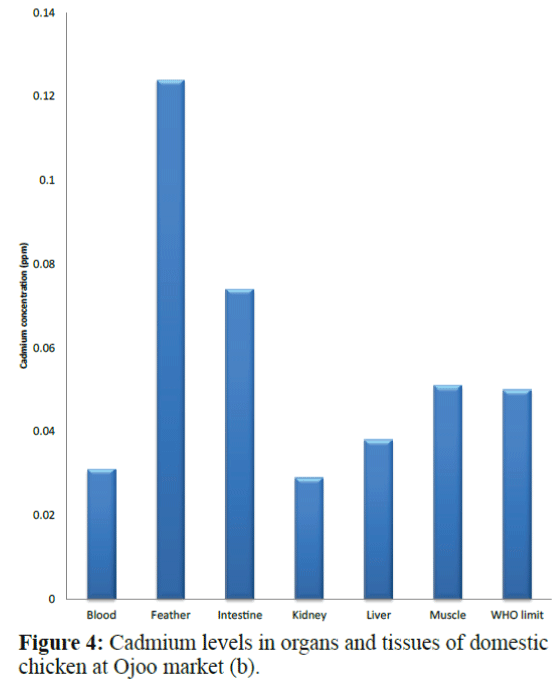
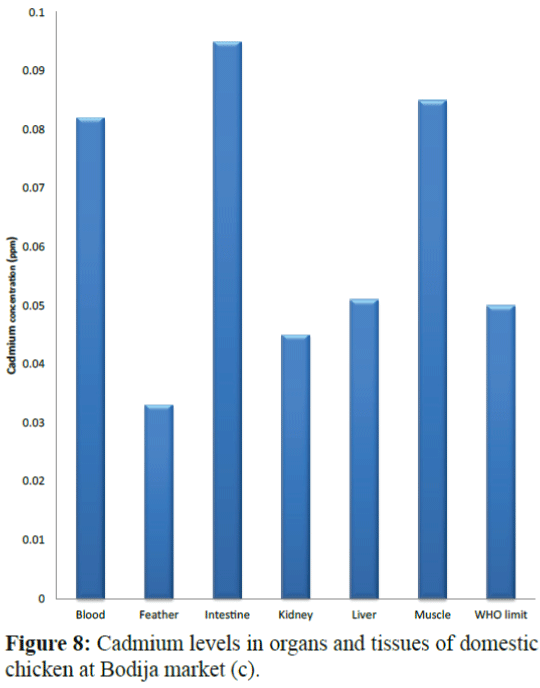
Results of the variations in the cadmium levels of chicken parts from the sampling sites (Bodija, Sango and Ojoo markets) are shown in Figure 1 to 10 below in comparison with World Health Organisation (WHO) guideline limit (WHO 2004). As shown in Figure 1, in sample A (Bodija market), blood (0.013 ppm), kidney (0.009 ppm) liver (0.014 ppm) and muscle (0.029 ppm) were lower than the WHO limit of 0.050 ppm while that of the feather (0.079 ppm) and intestine (0.067 ppm) were higher. Overall, the kidney had the lowest concentration (0.009 ppm) while the feather had the highest value (0.079 ppm). In sample B (Bodija market) shown in Figure 2, blood (0.015 ppm), kidney (0.001 ppm), liver (0.004 ppm) and muscle (0.049 ppm) were lower than the WHO limit of 0.050 ppm while that of the feather (0.106 ppm) and intestine (0.073 ppm) were higher than the WHO limit. Overall, the kidney had the lowest cadmium concentration (0.001 ppm) while the feather had the highest value (0.106 ppm). In sample C (Ojoo market) shown in Figure 3, blood (0.003 ppm), kidney (0.011 ppm), liver (0.049 ppm) were lower than the WHO limit of 0.050 ppm while that of the muscle (0.051 ppm), feather (0.109 ppm) and intestine (0.088 ppm) were higher than the WHO limit. Overall, the blood had the lowest cadmium concentration (0.003 ppm) while the feather had the highest value (0.109 ppm). In sample D (Ojoo market) shown in Figure 4, blood (0.031 ppm), kidney (0.029 ppm), liver (0.038 ppm) were lower than the WHO limit of 0.050 ppm while that of the muscle (0.051 ppm), feather (0.124 ppm) and intestine (0.074 ppm) were higher than the WHO limit. Overall, the kidney had the lowest cadmium concentration (0.029 ppm) while the feather had the highest value (0.124 ppm). In sample E (Sango market) shown in Figure 5, blood (0.032 ppm) was lower than the WHO limit of 0.050 ppm while that of the muscle (0.052 ppm), kidney (0.055 ppm), liver (0.053 ppm), feather (0.064 ppm) and intestine (0.078 ppm) were higher than the WHO limit. Overall, the blood had the lowest cadmium concentration (0.032 ppm) while the intestine had the highest value (0.078 ppm). In sample F (Sango market) shown in Figure 6, blood (0.027 ppm), feather (0.033 ppm) and muscle (0.044 ppm) were lower than the WHO limit of 0.050 ppm while that of the kidney (0.064 ppm), liver (0.057 ppm), and intestine (0.089 ppm) were higher than the WHO limit. Overall, the blood had the lowest cadmium concentration (0.027 ppm) while the intestine had the highest value (0.089 ppm). In sample G (Sango market) shown in Figure 7, kidney (0.049 ppm), feather (0.032 ppm) and were lower than the WHO limit of 0.050 ppm while that of the blood (0.052 ppm) liver (0.078 ppm), muscle (0.084 ppm) and intestine (0.090 ppm) were higher than the WHO limit. Overall, the feather had the lowest cadmium concentration (0.032 ppm) while the intestine had the highest value (0.090 ppm). In sample H (Bodija market) in Figure 8, kidney (0.045 ppm) and feather (0.033 ppm) were lower than the WHO limit of 0.050 ppm while that of the blood (0.082 ppm) liver (0.051 ppm), muscle (0.085 ppm) and intestine (0.095 ppm) were higher than the WHO limit. Overall, the feather had the lowest cadmium concentration (0.033 ppm) while the intestine had the highest value (0.095 ppm).
In sample I (Bodija market) in Figure 9, feather (0.041 ppm), liver (0.036 ppm) and intestine (0.031 ppm) were lower than the WHO limit of 0.050 ppm while that of the blood (0.077 ppm) kidney (0.079 ppm) and muscle (0.074 ppm) were higher than the WHO limit. Overall, the intestine had the lowest cadmium concentration (0.031 ppm) while the kidney had the highest value (0.079 ppm). In sample J (Bodija market) in Figure 10, kidney (0.010 ppm) and intestine (0.028 ppm) were lower than the WHO limit of 0.050 ppm while that of the liver (0.052 ppm) feather (0.081 ppm) blood (0.094 ppm) and muscle (0.073 ppm) were higher than the WHO limit. Overall, the kidney had the lowest cadmium concentration (0.010 ppm) while the blood had the highest value (0.094 ppm).
Health risk assessment
Calculating the health risk assessment using the Target Hazard Quotient (THQ) as restated below.

Where EF is exposure frequency (from 365 days per year for people who eat chicken meat 7 times a week to 52 days per year for people who eat chicken meat once a week)
ED is the exposure duration (70 years) equivalent to the average lifetime
FIR is the food ingestion rate (for egg: 37.2 g/person/day).
C is the metal concentration in for egg content (3.217 µg/g) in this case meat and offal
RFD is the oral reference dose (USEPA 1997, 2000)
WAB is the average body weight (64 kg, the reference weight for the age categories)
TA is the average exposure time for non-carcinogens (365 day/year * ED).

THQ = 1.865×10-3
If THQ value is equal to or greater than 1, an exposed population to the pollutant of concern (Cd) will be at health risk. Since the THQ value <1, therefore the general populace of Ibadan metropolis that feed on chicken meat from these markets will not be at health risk in the short-term. However, continuous consumption of the small doses of the cadmium in the chicken meat will lead to its bioaccumulation and consequent deleterious health effects.
Discussion
Cadmium level in the chicken meat and offal in ibadan
The high cadmium level observed in feathers (0.068 ± 0.03) at Bodija market could be as a result of continuous deposition of the heavy metal from the circulatory system during the developmental stages while the low levels in the kidney can be as a result of efficient excretion (Nagabhushanam et al., 1999; Iwegbue et al., 2008; Miller and Spoolman 2010). The high mean cadmium levels in the intestine (0.086±0.007) at Sango market can be attributed to the potential of the villi to retain some of the metal in its tissues (Oforka et al., 2012) while the low levels in the blood may be as a result of efficient uptake by tissues at the capillary level. Similarly, the high mean cadmium levels in the feathers (0.117 ± 0.011) from Ojoo market and the low concentration in the blood (0.017± are as a result of physiological differences in absorption, retention and excretion as stated above.
Variations in the cadmium levels in chicken at the sampling sites
Findings in sample A (Bodija market) where cadmium concentration in the blood, kidney , liver and muscle were lower than the WHO limit corroborates similar findings by Akan et al., 2010 in a study of chickens from Kasuwan Shanu market in Maiduguri that shows cadmium statistically significant in the kidney and liver but within tolerance limits. The study showed the cadmium range of 0.01-0.34 ± 0.23 ug/g (ppm) while this present study under discussion varied from 0.01 (Kidney)-0.094 (Blood). On the other hand, the high cadmium level in the feather which is not an edible part provides an excretory feature for storage and to get rid of the toxic heavy metal (Cd). No study on heavy metals in chicken parts has previously included feathers for assessment in Nigeria. More so, the intestine as a chicken part is not commonly consumed in most cultures and therefore the elevated cadmium (Cd) above WHO guideline limit does not pose a public health threat to the vast majority of the populace. Similarly, samples B, H I and J from Bodija market show the similar trend with the blood peaking in the cadmium concentration and the kidney having a lower level of the heavy metal. In Ojoo market, samples C and D, show that with the exception of intestine and kidney, all the other organs exceeded WHO limit which is in agreement with studies by Onianwa et al., (2000), Okoye et al., (2015) and Oforka et al., (2015) in whose study cadmium exceeded the permissible WHO level in the liver and kidney of about 10-405 of the sample. In Sango market, samples E, F and G show that with the exception of intestine and kidney, all the other organs exceeded WHO limit which is in agreement with studies by Okoye et al., (2015) in whose study cadmium exceeded the permissible WHO level in the liver and kidney of about 10-405 of the sample.
Health risk associated with cadmium levels in the chicken meat and offal in ibadan
Most of the chicken meat and offal from Ibadan had cadmium concentration above FAO/WHO recommended limit of 0.05 ppm thereby indicating that consumers of these animal products are at health risk in the long term (FAO/ WHO 1993 and WHO, 1989). Although, the target hazard quotient (THQ) is <1 which is indicative of no health risk, the continuous consumption of the contaminated chicken meat and offal could lead to its bioaccumulation and the advent of potential health hazards associated with the toxic metal. This implies that consumers of this contaminated meat must combine natural chelating agents like fruits and vegetables in their diet mostly those rich in ascorbic acid. The chelating agents would naturally facilitate the excretion of cadmium in a harmless form in the urine.
Conclusion
From this study, it can be seen that cadmium being a toxic metal can bioaccumulate in the human body and have debilitating effects on the body. This study is aimed at calling the attention of scholars and the general populace to its adverse effects. Since the values of Cd were lower in the liver and kidney which are the main targets for Cd accumulation in several organisms, more research work is needed to analyze the feeds and fluids administered to the chicken in this study whether they contain chelating agents such as ascorbic acids that aid in the excretion of Cd, or natural physiological ability to get rid of the toxin. The environmental fate and the toxicity of cadmium calls for a global initiative aimed at minimising human and environmental consequences of the ongoing cadmium emissions. The relevance of considering a global initiative comes, furthermore, from the fact that cadmium used intentionally in products is traded globally and that effective risk reduction measures thus must be seen in a global context. Caution is required in the disposal of cadmium-rich wastes such as electronic wastes (e-wastes) in order to regulate the quantity of the heavy metal that will become bioavailable in the human environment.
Funding
This study was supported by a laboratory space from Department of Zoology, University of Ibadan, Ibadan, Nigeria.
Conflicts of Interest
Authors report no conflict of interest in relation with this study.
Authors’ Contributions
Emmanuel drafted the manuscript which was reviewed by a colleague. Christianah digested the samples for analyses under the guidance and supervision of Emmanuel. Vivian collected the samples for the study and pulverized them. All of the data was obtained through analyses using facilities in the Department of Agronomy, University of Ibadan, Nigeria.
Acknowledgement
The lab space provided by Department of Zoology, University of Ibadan for the study is hereby acknowledged.
References
- Akan, J.C., Abdulrahman, F.L., Sodipo, A.L. andChiroma, Y.A., (2010). Distribution of Heavy Metals in the Liver, Kidney and Meat of Beef, Mutton, Caprine and Chicken from KasuwanShanu Market in Maiduguri Metropolis, Borno State, Nigeria. Research Journal of Applied Sciences, Engineering and Technology,2: 743-748.
- ATSDR.,(2005). Toxicological Profile for Cadmium.Agency for Toxic Substance and Disease Registry, U.S.
- FAO/WHO., (1993). Evaluation of certain food additives and contaminants, WHO Technical Report Series 837. Geneva.
- Iwegbue, C.M.A., Nwajei, G.E. andIyoha, H., (2008). Heavy metal residues of chicken meat and gizzard and turkey meat consumed in Southern Nigeria.Bulgarian Journal of Veterinary Medicine, 11: 275-280.
- Miller, T.G. andSpoolman, S.C., (2010).Environmental Science.13th Edition and International Edition.Brooks and Cole, California, USA.
- Nagabhushanam, R., Kodarkar, M.S. andSarojini, R., (1999). Textbook of Animal Physiology.2nd Edition.IBH Publishing Co.PVT.Ltd.India.
- Nriagu, J.O., (1988). A silent killer in environmental metal poisoning. Environmental Pollution, 50: 139-161.
- Nriagu, J.O., (1980). Cadmium in the Atmosphere and in Precipitation. In: Cadmium in the Environment, Part 1, Ecological Cycling. John Wiley &Sons :71-114.
- Nriagu, J.O., (1989).A Global Assessment of Natural Sources of Atmospheric Trace Metal.Nature., 338: 47-49
- Nriagu, J.O. andPacyna, J.M., (1988). Quantitative Assessment of World-wide Contamination of Air, Water and Soils by Trace Metals. Nature., 333: 134-139.
- Oforka N.C., Osuji, L.C. andOnwuachu, U.I., (2012). Assessment of Heavy Metal Pollution in Muscles and Internal Organs of Chickens Raised in Rivers State, Nigeria.Journal of Emerging Trends in Engineering and Applied Sciences (JETEAS), 3: 406-411.
- Okoye, P.A.C., Ajikwe, V.I.E., Okeke, O.R., Ujah, I.I., Asalu, U.B. andOkeke, D.O., (2015). Estimation of Heavy Metal Levels in the Muscle, Gizzard, Liver and Kidney of Broiler, Layer and Local (Cockerel) Chickens Raised within Awka Metropolis and Its Environs, Anambra State, South Eastern Nigeria.Journal of Environmental Protection., 6: 609-613.
- Olusola, A.V., Belleh, E.D. andAyoade, O.I., (2008). Assessment of tetracycline, lead and cadmium residues in frozen chicken vended in Ibadan and Lagos, Nigeria.Pakistan Journal of Biological Sciences, 15: 839-844.
- Onianwa, P.C., Lawal, J.A., Ogunkeye, A.A. andOrejimi, B.M., (2000). Cadmium and nickel composition of Nigerian foods. J Food Compos Anal., 13: 961-969.
- Suttle, N.F., (2010). Mineral Nutrition of Livestock.4th edition CAB International, Oxfordshire, UK.
- Pacyna, J.M. andPacyna, E.G., (2001).An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide.Environmental Reviews, 9: 269-298.
- Pond, W.G., Church, D.C. and Pond, K.R., (1995).Basic Animal Nutrition and Feeding.4th Edition. John Wiley & Sons, Inc., New York, NY, USA.
- WHO.,(1989). Evaluation of Certain Food Additives and Contaminant. Thirty-third Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 776. World Health Organization.