- International Journal of Pure and Applied Zoology (2016) Volume 4, Issue 2
Elm Bark Beetle Population Dynamics and its Attack Density in Relation to Host Tree Diameter, Maternal Gallery Length and Oviposition in the Elm Nursery Stands
- Corresponding Author:
- Abdul Lateef Khanday
Postgraduate Department of Zoology, University of Kashmir, Hazratbal, Srinagar ? 190006 India
E-mail: lateefkhanday@gmail.com
Received 06th April 2016; Accepted 07th May 2016; Published 17th May 2016
Abstract
Elm bark beetle Scolytus kashmirensis is a key pest on Ulmus villosa in the social forestry ecosystem of Kashmir. The population dynamics, effects of attack density on the gallery length and oviposition and importance of log diameter on emergence of the beetle pest were determined in the present study. The results showed that seasonal fluctuation in population of immature scolytids correlated with adult population. The seasonal peaks in population density of immature scolytids and adults have a direct influence on one another. The adult beetles arrived in April up to June with peak period during the first ten days of May (2.20 ± 0.78 SD per 0.2 m2 area). The second arrival period started from July and extended up to September with peak period during the last ten days of July (3.40 ± 0.51 SD per 0.2 m2 area). The logs infested by parent beetles (of partial 2nd generation) during April to May (2013) started producing adults of 1st generation from July onwards with peak period during the first ten days of July (11.90 ± 2.02 per 0.2 m2 area). The logs infested by beetles from July to September (2013) did not show any emergence and the 2nd generation adults emerged from April of the following year which continued up to June with peak period during the middle of May (8.50 ± 2.32 SD per 0.2 m2 area). The results of attack density on gallery length and oviposition showed a significant positive correlation between the variables. In our study, we also evaluated relationship between log diameter and emergence of beetles. In addition, the optimal attack densities and influence of log diameter on beetle population are discussed.
Keywords
Population dynamics, Scolytus kashmirensis, arrival sequence, attack density, Ulmus villosa
Introduction
Bark beetle population dynamics is primarily influenced by the main factors such as host tree availability and quality, while other factors such as parasitism or predation are usually assigned a minor role (Reeve, 1997). Unlike most insects, the reproduction of bark beetles is entirely dependent upon the death of all, or part, of its host (Shepherd, 1966; Wood, 1972). Their population, particularly those species which colonize living trees, is often characterized by dramatic changes in abundance. Their boring activities in the internal tissues of the tree, however, initiate host chemical and histological responses which inhibit beetle development and survival (Shrimpton & Whitney, 1967; Reid & Shrimpton, 1971; Wong & Berryman, 1977). Due to their continued attack on selected trees, these beetles are capable of exhausting the host’s defensive response (Berryman, 1976).
Bark beetles are among the main insect pests, which have created a perennial problem in the management of conifer and broad-leave tree species of Kashmir valley in the recent years. Ulmus villosa which is grown as agro-forestry and social forestry tree in Kashmir is attacked by many pests and diseases, among them the bark beetle Scolytus kashmirensis Schedl is considered as a serious pest found only in the Himalayas (Schedl, 1957). Some of its aspects including its immature development, life history and behavior have been analyzed in detail (Buhroo, 2012; Khanday & Buhroo, 2015). After mating, S. kashmirensis makes a maternal gallery which is fairly species specific. There are five larval instars during its development and the larvae feed under bark. After the completion of their development mostly in 40-46 days, adults emerge and colonize healthy or stressed plants. Pupation takes place for 10-20 days and adults live for 45-60 days. Most of the bark beetle species breed in slash, broken, fallen, dying, or large limbs of trees but some are capable of primary attack on healthy trees when conditions are favorable (Wood, 1982; Lieutier, 2004). Bark beetles prefer older elms for feeding and therefore much more likely to become diseased compared with young trees (Sengonca & Leisse, 1984). The incidence of bark beetles is largely determined by the distribution and abundance of their host tree species and climate (Lekander, 1977). There are reports that bark beetles and pathogens interact to cause expensive losses in forest trees e. g., Ophiostoma himal-ulmi, the Dutch elm disease pathogen occurring in the Himalayan region (Brasier & Mehrotra, 1995), is transmitted by S. kashmirensis.
A preliminary analysis of population dynamics of S. kashmirensis is crucial to understand the seasonal fluctuations in population and thus could be useful to predict its arrival sequence and infestation because there are still considerable gaps in our knowledge regarding such aspects. Moreover, such studies will be the prerequisite to understand its intraand interspecific associations and finally to develop an efficient pest management system.
The aim of this study was to investigate in detail the arrival sequence and population structure, effects of attack density on the maternal gallery length and oviposition and importance of log diameter on emergence of the elm bark beetle.
Materials and Methods
Study sites
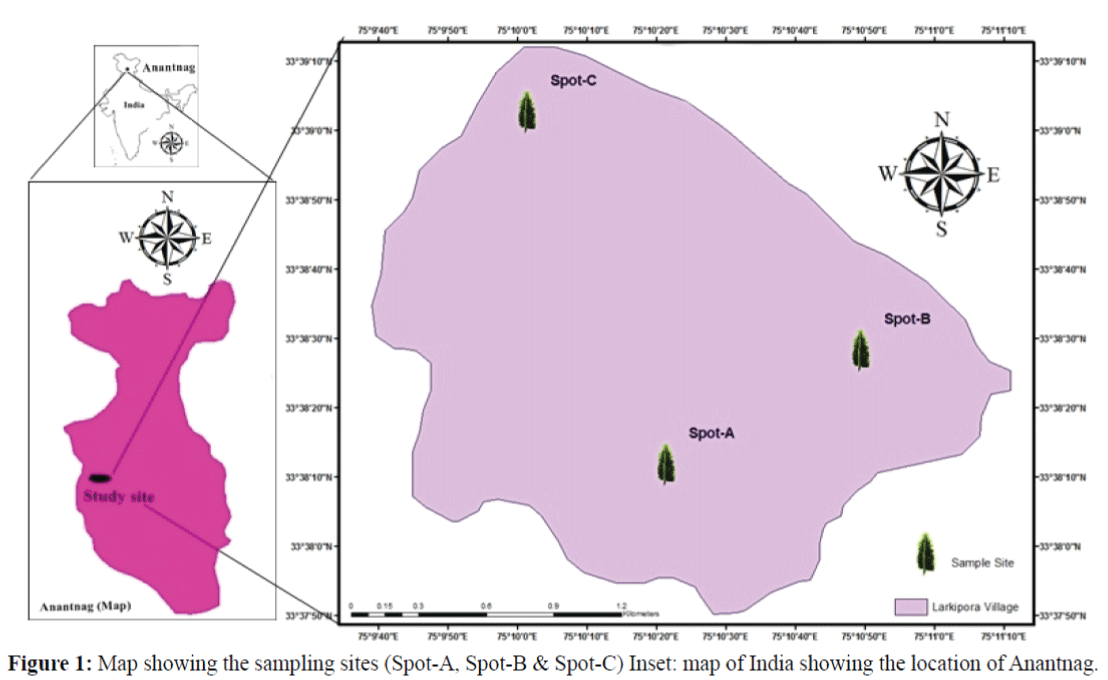
This study was conducted from 2013 to 2014 at a village “Larkipora” GPS position (330 38.738’ N, 075010.060’E and Elevation 5482 feet) in district Anantnag where elm trees (Ulmus villosa) are found in abundance. In order to estimate the population dynamics of S. kashmirensis we conducted preliminary field surveys in March 2013 to assess the number and condition of trees attacked by elm bark beetle at the study area. Based on the preliminary surveys of elm beetle infestations, three sites were chosen and marked as spot A, spot B and spot C (Figure 1). Spatial information regarding sample sites were recorded in the form of latitude and longitude with the help of handheld GPS (Garmin eTrex 10) and then file was exported to Esri ArcGIS 9.2.1 software. Subsequently data was coregistered with cartosat-1 satellite data to develop the layout.
Sampling procedure
Sampling procedures for monitoring seasonal beetle population density were adopted as per the earlier studies on Scolytus nitidus (Buhroo, 2004). Estimation of seasonal fluctuation in beetle population on elm logs was made by (1) counting population density of immature scolytids present under bark (2) counting entrance holes on infested logs and (3) counting emerging holes on infested logs throughout the season.
The observation related to counting population density of immature scolytids was conducted during 2013-14. In first week of March 2013, fresh logs were placed at each spot at a distance of 4ft around the trunk of the infested live tree with clear symptoms of bark beetle attack followed by the installation of few new fresh logs after every month in the same fashion throughout the season. A wooden frame of 0.07 m2 area has been made so as to mark bark area which represents the population sample of immature scolytids under bark throughout the season. After every ten days the bark area (0.07 m2) was dissected very carefully (one from each spot) and the number of individuals was counted from all three spots. The number of immature scolytids was also separated by the stage of life and the numbers of individuals in each stage were counted in order to know the relative proportion of each stage. The same observations were repeated throughout the year in order to determine the peak and decline seasons of their population.
The experiment related to the arrival sequence of S. kashmirensis was conducted during first week of March 2013 on same stands but separate logs were installed in the above mentioned fashion. The relative number of beetles that land on experimental logs was recorded by counting the number of entrance holes per 0.2 m2 after every ten days and encircled by green marker pen so as to avoid recounting of entrance holes. A different set of logs was installed for counting entrance holes in case of 1st generation so as to avoid reselection of logs used for overwintering generation. The emerging holes perforated by beetles per 0.2 m2 on selected logs were counted and encircled by red marker pen. The number of holes girdled at a particular time was taken as a measure for population count of adult beetles for that period. The numbers of emerging holes per 0.2 m2 on selected logs were counted after every ten days until emergence terminated.
Sampling procedures used for observing effects of attack density on gallery length and oviposition and importance of log diameters on emergence were identical to those adopted by Zhang, (1992). In each sample log the attack density (attacks per 0.2 m2 area), maternal gallery length, and eggs deposited by parent beetles were recorded. Emergence holes were counted per 0.2 m2 bark area from selected logs whose diameters range from 5 to 80 cm.
Data analysis
Observations made during the present study were tabulated and graphically presented as per the required statistical methods. Data after counting population density of immature scolytids, attacking holes and emergence holes were summarized as mean ± standard deviation (SD). Quadratic regression was performed on the data to determine the relationship between variables. Statistical analyses of data were carried out with SPSS 11.5 (SPSS Inc., Chicago, IL, USA).
Results
Population density of immature stages
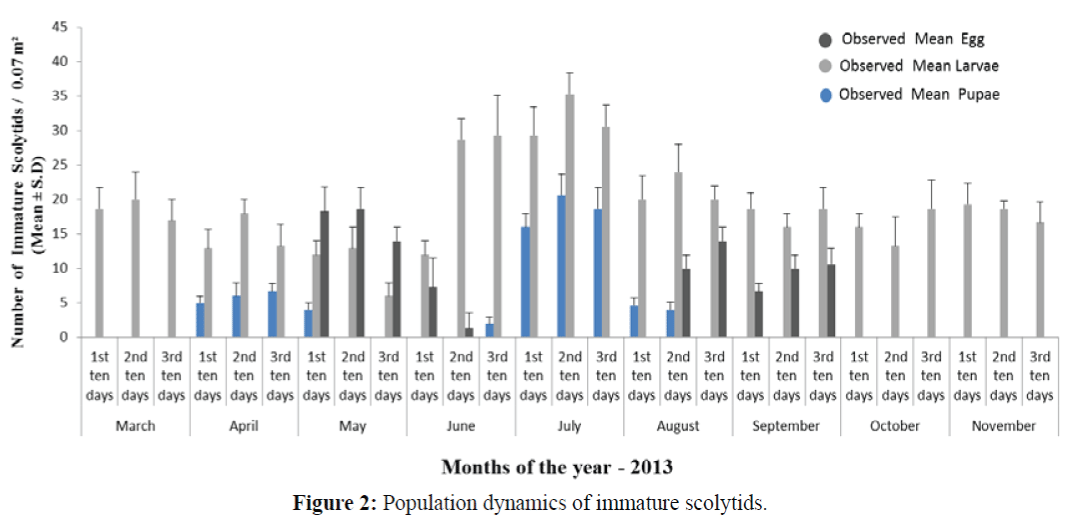
The data regarding the population counts of immature stages of S. kashmirensis is shown in Figure 2. The data indicates that pupal development of 2nd generation (partial one) starts from April and may extend up to May with peak period during the last ten days of April (6.66 ± 1.15 SD). The densities of pupae can be correlated with the number of emerging adults leading to 1st generation. The eggs of the 1st generation were laid during the month of May and extend up to June with peak period during the middle days of May (18.66 ± 3.05 SD). The initial larvae of 1st generation were observed during May and extend up to July with peak season during the middle days of July (35.33 ± 3.05 SD). The pupal period of the 1st generation lasts from June to August with peak period during the 2nd ten days of July (20.66 ± 3.05 SD). After the emergence of 1st generation adults, eggs of the 2nd generation were observed during August and September with peak period during the last ten days of August (14.00 ± 2.00 SD). The larval period of the 2nd generation (partial one) starts from July which extends up to May of the following year. These studies suggest that the different peaks in population of immature stages of S. kashmirensis can be correlated to optimum conditions required for each developmental stage.
Arrival sequence of adults
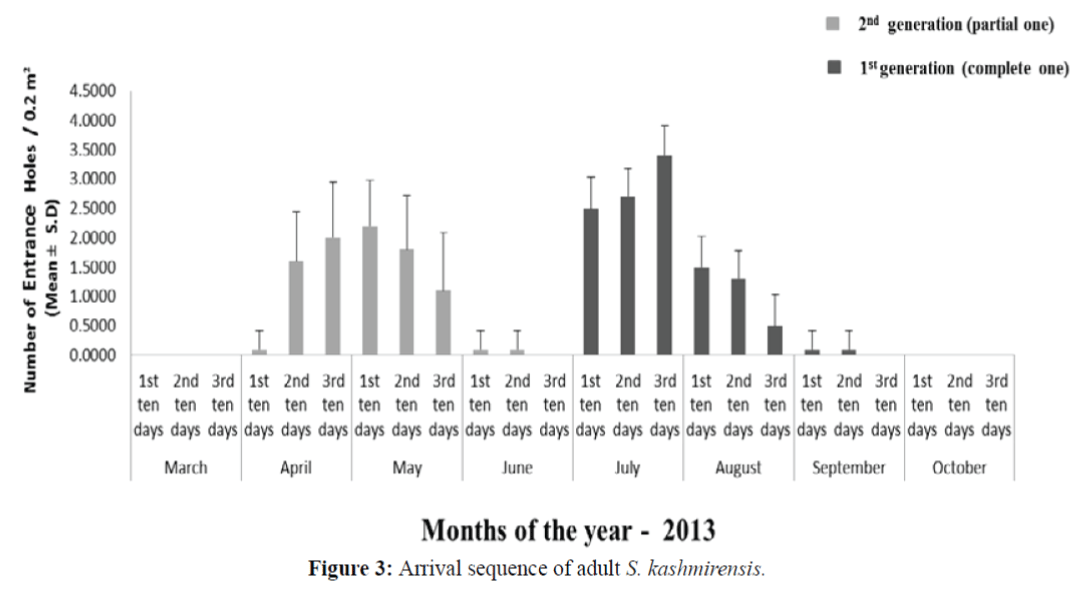
The data based on the observations of mean number of entrance holes (Figure 3) shows that adults of S. kashmirensis arrived in April and extend up to June with peak period during the first ten days of May (2.20 ± 0.78 SD). The data also reveals their presence from July up to September with peak period during the last ten days of July (3.40 ± 0.51 SD). There were little attacks in September beyond which no entrance holes were observed.
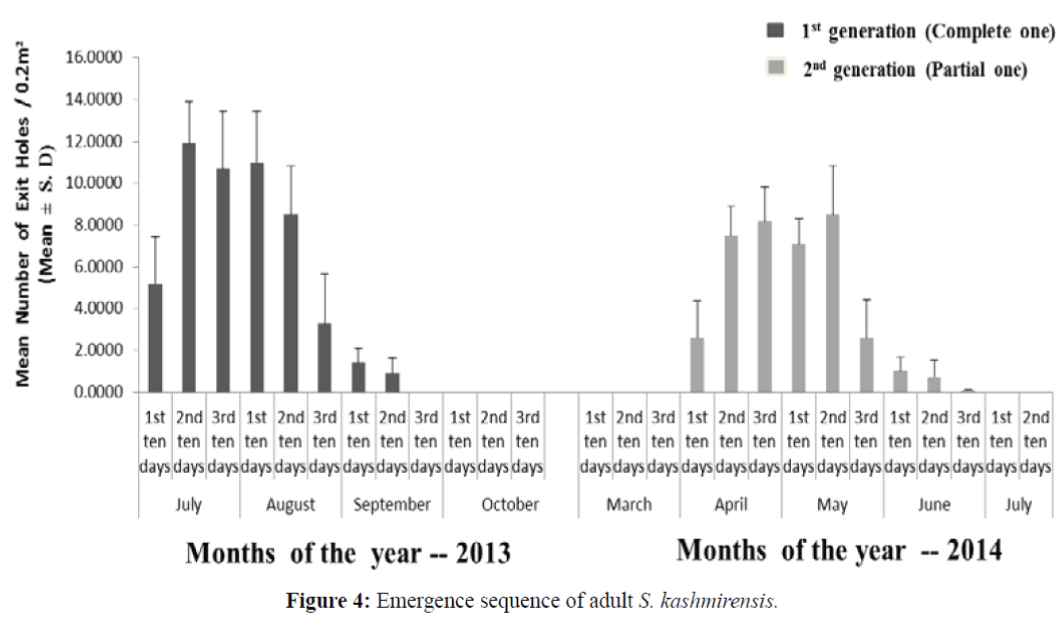
The data based on mean number of exit holes (Figure 4) also shows that the logs infested by parent beetles (of partial 2nd generation) during April to May (2013) started producing adults of 1st generation from July onwards with peak period during the first ten days of July (11.90 ± 2.02). The logs infested by beetles from July to September (2013) does not show any emergence and the 2nd generation adults emerged from April of the following year which continued up to June with peak period during the middle of May (8.50 ± 2.32 SD).
Effects of attack density on gallery length and oviposition
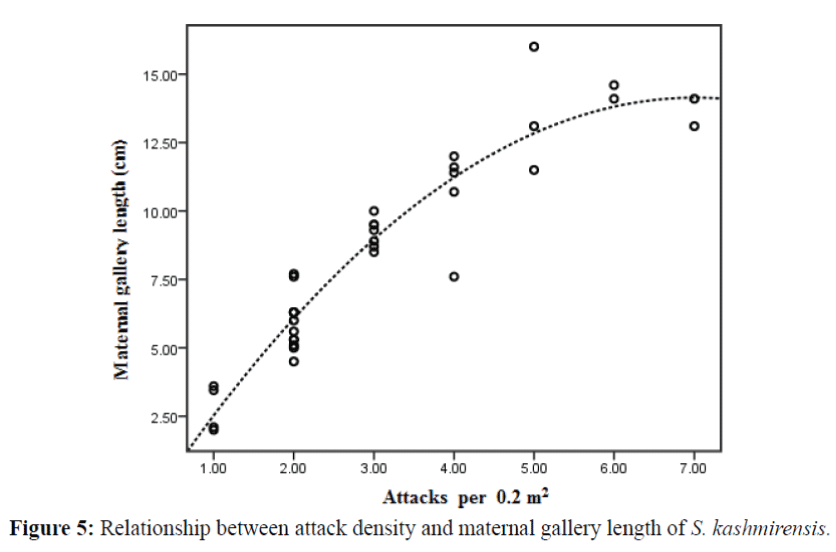
Data based on effects of attack density on maternal gallery length and eggs deposited by parent beetles are summarized in table 1. The range of attack densities per 0.2 m2 area on selected logs was from 1 to 7 attacks (Figures 5 and 6) and the length of maternal galleries and eggs deposited by female beetles ranged from 2 to 16 cm and 12 to 110 respectively (Figures 5 and 6). This variation in attack density allows us to describe the relationship between the variables. Significant quadratic relationships were found in regression analyses of (i) attack density vs. maternal gallery length and (ii) attack density vs. eggs deposited by female beetles as shown in table 1 and Figures 5 and 6.
| S. No. | Data set | N | Model | R2 | P- value |
|---|---|---|---|---|---|
| 1 | Attack per 0.2 m2 vs. Maternal gallery length |
35 | y=- 0.321x2 +4.504x–1.653 | 0.908 | <0.001 |
| 2 | Attack per 0.2 m2 vs. Eggs per gallery |
35 | y=- 2.530x2+4.355x–14.975 | 0.893 | <0.001 |
| 3 | Log diameter vs. Emergence per 0.2 m2 |
145 | y=-0.002x2+0.290x+21.551 | 0.739 | <0.001 |
Table 1: Results of quadratic regression models used to analyze relationship between variables.
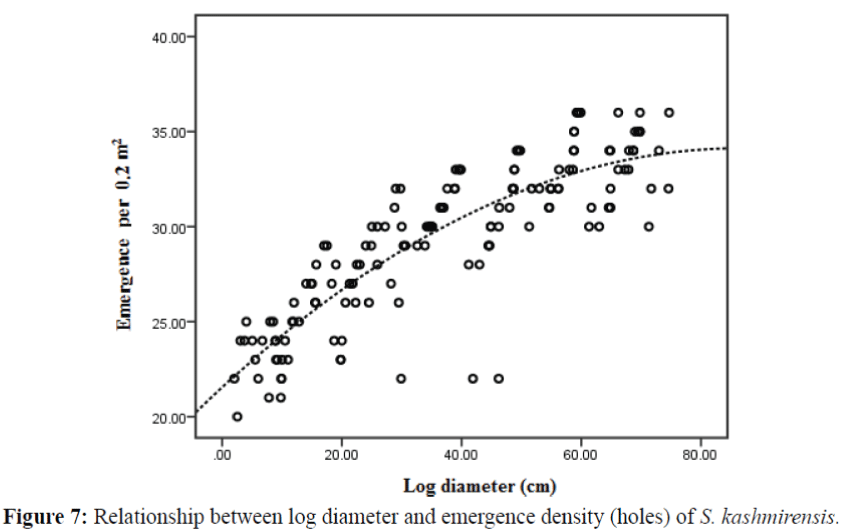
Relationship between log diameter and emergence density
The result of emergence density vs. log diameters is summarized in Table 1. The emergence density increased in quadratic relationship as the diameter of the log increased indicating a significant positive relationship between the two variables (Figure 7). These results demonstrate that emergence is also directly influenced by log diameter in the absence of host resistance. Maximum emerging holes per 20 cm2 area were observed when log diameter reached 60 cm. This can be considered an optimal log diameter for individuals in a population on the bark area. When log diameter increased beyond 60 cm, the decline of the curve might be explained by the fact that the bark area (20 cm2) had been totally explored by oviposition galleries and consequently, the construction of maternal galleries was impossible due to lack of free space.
Discussion
The present observations showed that S. kashmirensis swarmed from middle of April up to September with two peak periods during May and July-August in Kashmir. The beetles of S. scolytus on elm swarmed twice in a year, the first swarming being as a rule in May and the second in July or August, according to location in North America (Novak, 1976). In central Europe, there were two flight periods of S. mali on apple trees, the first flight occurring during May-June and the second in July-August (Rudinsky, 1978). Three swarming peaks of S. nitidus on apple trees in Kashmir have been reported (Buhroo, 2004).
The populations of S. kashmirensis are able to kill and attack living trees at densities which allow optimal survival of their offsprings. Also, it has been reported that most of the change in total live population occurs in the 4th and 5th instar larvae and the size of the 1st generation, at least, depends on mortality in these stages (Beaver, 1966). However, the other beetle pest species (Cryphalus sp.) was also noticed on the same host during the present study. The host thus provides shelter and food to its entire pest species which enter in intraand interspecific competition. Rudinsky (1962) reports that intra- and interspecific competition for food and space is a major mortality factor limiting within-tree populations.
The present results demonstrate that maternal gallery length and egg deposition are directly influenced by attack density in the absence of host resistance. The maximum length of maternal galleries (14 cm) and eggs deposited (100) were observed when attack density reached 6 attacks per 0.2 m2 area. This can be considered an optimal attack for individuals in a population on the bark area. The decline of maternal gallery length and oviposition with increasing attack density is partially due to intraspecific competition between parent adults for limited breeding space such that females have insufficient area for gallery extension. S. kashmirensis is larger in size (4 mm long) among Scolytus species so it is expected that relatively less progeny could be supported by the phloem than for smaller sized bark beetles. There are reports of intraspecific competition at high breeding densities which results in shorter maternal galleries and thus reduced oviposition (Anderbrant, 1990; Weslien, 1994). It has been shown that offspring number per female decreases at higher attack densities (Miller & Keen, 1960; McMullen & Atkins, 1961; Anderbrant, 1985).
Bark thickness and log diameter act as the other functional characteristics by which females make oviposition decisions and thus decide within-tree insect populations. The present results have shown that the optimal log diameter for maximum population on the bark area is 60 cm. The importance of log diameter on host reproduction has been noted for a number of bark beetle species (Parker & Stevens, 1979). However, Wesley (1995) found that Arizona five-spined Ips in southern Arizona prefer 10 cm diameter logs over 5 or 20 cm logs. Diameter has also been noted as the primary factor predicting host selection by Douglas-fir beetle, Dendroctonus pseudotsugae in Douglas-fir, Pseudotsuga menziesii (Mirbel) Franco (Reid & Glubish, 2001). Beetles ultimately prefer large trees due to positive relationship between tree diameter and phloem thickness (Amman, 1969; Shrimpton & Thomson, 1985). In trees with thick phloem, beetles lay more eggs per centimeter of gallery, experience less intraspecific competition of larvae and ultimately produce larger brood beetles compared to beetles in trees with thin phloem (Amman & Cole, 1983; Cole, 1973; Amman & Pace, 1976).
It must be pointed out that the conclusions in the present study were obtained only in the absence of host resistance. When the beetles attack living trees at relatively low densities, the eggs and larvae face a risk of being killed by the exudation of resin, and thus one could expect the survival to increase with density up to a point determined by the vigour and resin flow of the tree, as indicated by Berryman (1974) and Raffa & Berryman (1983). However, the effects of density on offspring production in S. kashmirensis in standing, live trees remain unknown.
Conclusion
Bark beetles use the inner bark of their host trees as food and substrate for rearing their young. The genus Scolytus not only introduces fungi into tree tissues but other pathogens as well that often lead to the trees’ death. Although certain aspects of population dynamics and effects of attack density of this elm bark beetle has been worked out in the present study but much needs to be done regarding the parameters like effects of density on offspring production etc. The Himalayan region could harbor new biodiversity of Scolytine species that demands further exploration of the insect–plant interactions for efficient management of the forest ecosystem.
References
- Amman, G.D., (1969). Mountain pine beetle emergence in relation to depth of lodgepole pine bark. USDA Forest Service, Intermountain Forest and Range Experiment Station, Ogden, UT, Research Note INT-96.
- Amman, G.D. and Cole, W.E., (1983). Mountain pine beetle dynamics in lodgepole pine forests. Part II: population dynamics. USDA Forest Service, Intermountain Forest and Range Experiment Station, General Technical Report INT-145: 59.
- Amman, G.D. and Pace, V.E., (1976). Optimum egg gallery densities for the mountain pine beetle in relation to lodgepole pine phloem thickness. USDA Forest Service, Intermountain Forest and Range Experiment Station, Ogden, UT, Research Note INT-209: 8.
- Anderbrant, O., (1990). Gallery construction and oviposition of the bark beetle, Ipstypographus (Coleoptera: Scolytidae) at different breeding densities. Ecological Entomology, 15: 1-8.
- Anderbrant, O., Schlyter, F. andBirgersson, G., (1985). Intraspecific competition affecting parents and offspring in the bark beetle. Ipstypographus. Oikos., 45: 69-98.
- Beaver, R.A., (1966). The development and expression of population tables for the bark beetle Scolytusscolytus (F). Journal of Animal Ecology, 35: 27-41.
- Berryman, A.A., (1974). Dynamics of bark beetle population: towards a general productivity model. Environmental Entomology, 3: 579-585.
- Berryman, A.A., (1976). Theoretical explanation of mountain pine beetle dynamics in lodge pole pine forests. Environmental Entomology, 5: 1225-1233.
- Brasier, C. andMehrotra, M.D., (1995).Ophiostomahimal-ulmi sp. nov., a new species of Dutch elm disease fungus endemic to the Himalayas. Mycological Research, 99: 205-215.
- Buhroo, A.A., (2012) Host selection behavior and incidence of bark beetle Scolytuskashmirensis (Coleoptera: Curculionidae: Scolytinae) attacking elm trees (Ulmus spp.) in Kashmir. Forestry Studies in China,14: 224-228.
- Buhroo, A.A., Chishti, M.Z. andMasoodi, M.A., (2004). Studies on the population dynamics of shot-hole borer ScolytusnitidusSchedl (Coleoptera: Scolytidae). The Indian Forester, 130: 1451-1458.
- Cole, W.E., (1973). Crowding effects among single-age larvae of the mountain pine beetle, Dendroctonusponderosae (Coleoptera: Scolytidae). Environmental Entomologist, 2: 285-293.
- Khanday, A.L. andBuhroo A.A., (2015). Life history and biology of the elm bark beetle ScolytuskashmirensisSchedl (Coleoptera: Curculionidae: Scolytinae) infesting Ulmusvillosa in Kashmir. Open Journal of Forestry, 5: 443-453.
- Lekander, B., Bejer-Petersen, B., Kangas, E. and Bakke, A., (1977). The distribution of bark beetles in the Nordic countries. ActaEntomologicaFennica, 32: 1-37.
- Lieutier, F., Day, K.R., Battisti, A., Gregoire, J.C. and Evans, H.F., (2004). Bark and wood boring insects in living trees in Europe, A synthesis. In: Knizek, M. and R. Beaver, (Eds.), Taxonomy and Systematics of bark and ambrosia beetles. Kluwer Academic Publisher, Netherlands: 41-54.
- McMullen, L.H. and Atkins, M.D., (1961). Intraspecific competition as a factor in the natural control of the Douglas-fir beetle. Forest Science, 7: 197-203.
- Miller, J.M. and Keen, F.P., (1960). Biology and control of the western pine beetle. United States Department of Agriculture. Miscellaneous Publications No. 800. Washington DC.
- Novak, H.S., (1976). Atlas of Insects Harmful to Forest Trees.Vol. I. Elsevier Scientific Publishing Company. 335 Jan van Galenstraat P. O. Box 211 Amsterdam, The Netherlands.
- Parker, D.L. and Stevens, R.E., (1979). Mountain pine beetle infestation characteristics in ponderosa pine, Kaibab Plateau, Arizona, 1975-1977 (Vol. 367). Rocky Mountain Forest and Range Experiment Station, Forest Service, US Department of Agriculture.
- Raffa, K.F. and Berryman, A.A., (1983) The role of host plant resistance in the colonization behavior and ecology of bark beetles (Coleoptera: Scolytinae). Ecological Monographs, 53: 27-49.
- Reeve, J.D., (1997). Predation and bark beetle dynamics. Oecologia,112: 48-54.
- Reid, M.L. andGlubish, S.S., (2001). Tree size and growth history predict breeding densities of Douglas-fir beetles in fallen trees. The Canadian Entomologist, 133: 697-704.
- Reid, R.W. andShrimpton, D.M., (1971) Resistant response of lodge pole pine to inoculation with Europhiumclavigerum in different months and at different heights on stem. Canadian Journal of Botany,49: 349-351.
- Rudinsky, J.A., (1962). Ecology of Scolytidae. Annual Review of Entomology, 7: 327-348.
- Rudinsky, J.A.,Vallo, V. andRyker, L.C., (1978). Sound production in Scolytidae: Attraction and stridulation of Scolytusmali (Coleoptera: Scolytidae). ZeitschriftfürAngewandteEntomologie 86: 361-391.
- Schedl, K.E., (1957). Indian bark and timber beetles I. Indian Forest Records Entomology, 9: 165-169.
- Segonca, C. andLeisse, N., (1984). Significance of bark beetles (Coleoptera: Scolytidae) in the spread of Dutch elm disease in the area Euskirchen West Germany. ZeitschriftfürAngewandteEntomologie, 98: 413-423.
- Shepherd, R.F., (1966). Factors influencing the orientation and rates of activity of Dandroctonusponderosae Hopkins (Coleoptera: Scolytidae). Canadian Entomologist, 98: 507-518.
- Shrimpton, D.M. and Thomson, A.J., (1985). Relationship between phloem thickness and lodgepole pine growth characteristics. Canadian Journal of Forest Research, 15: 1004-1008.
- Shrimpton, D.M. and Whitney, H.S., (1967). Inhibitors of growth of blue stain fungi by wood extractives. Canadian Journal of Botany, 46: 757-761.
- Wesley, V.S., (1995). Effects of log diameter, length, and aspect on Ips brood production in ponderosa pine slash. M.S. thesis, Northern Arizona University, Flagstaff.
- Weslien, J., (1994). Interactions within and between species at different densities of the bark beetle Ipstypographus and its predator Thanasimusformicarius. EntomologiaExperimentalisetApplicata, 71: 133-143.
- Wong, B.L. and Berryman, A.A., (1977). Host resistance to the fir engraver beetle. Lesion development and containment of infection by resistant Abiesgrandis inoculated with Trichosporiumsymbioticum. Canadian Journal of Botany, 55: 2358-2365.
- Wood, D.L., (1972). Selection and colonization of ponderosa pine by bark beetles. Pages 101-107. In H. F. van Emden (ed.), R. E. S. Symposium No.6: Insect/Plant Relationships. Blackwell Scient. Publ. Oxford.
- Wood, S.L., (1982). The bark beetles and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Brigham Young University, Provo, UT.
- Zhang, Q.H., Byers, J.A. andSchlyter, F., (1992). Optimal attack density in the larch bark beetle, Ipscembrae (Coleoptera: Scolytidae).Journal of Applied Ecology, 29: 672-678.