Research Article - International Journal of Pure and Applied Zoology (2016) Volume 4, Issue 2
Effects Of The Exposure To Atrazine And Glyphosate Throughout Incubation On Bone Development Of Podocnemis Expansa (Testudines, Podocnemididae)
| Juliana dos Santos Mendonça1, Lucélia Gonçalves Vieira1, Sady Alexis Chavauty Valdes2*, André Luiz Quagliatto Santos1 1Wild Animal Research and Teaching Laboratory (LAPAS), Universidade Federal de Uberlândia (UFU), Uberlândia, MG, Brazil 2Centro Universitário de Patos de Minas (UNIPAM), Patos de Minas, MG, Brazil |
| Corresponding Author: Sady Alexis Chavauty Valdes E-mail: sadyzola@uol.com.br |
| Received 08th January 2016; Accepted 12th February 2016; Published 18th February 2016 |
Abstract
Brazil is considered to be one of the largest consumers of pesticides in the world, and herbicides represent the most commonly used class of these products. Atrazine and glyphosate, the most important herbicides, contaminate the waters of several Brazilian rivers and are involved in organ and bone malformations in different species. Among the possible target organisms, reptiles may be indirectly affected by pesticide use once their natural habitats are rivers and streams. Therefore, the objective of this study was to assess the possible effects of exposure to the herbicides atrazine and glyphosate in bone ontogeny of Podocnemis expansa. Eggs were artificially incubated in sand moistened with water contaminated with atrazine at concentrations equal to 0, 2, 20, or 200 μg/L, and glyphosate at 65, 650 or 6,500 μg/L. In the control group, the substrate was moistened with distilled water. Two eggs were collected from each incubator every ten days until hatching. For the analysis of bone development, soft tissues were diaphanized, and bones and cartilages were stained with Alizarin red S and Alcian blue, respectively. Specimens were analyzed by stereomicroscopy. Morphological characteristics of the cartilages and bones of the embryos were compared with descriptions of normal ontogeny available in the literature for P. expansa embryos. No abnormalities were observed in bone ontogeny of any of the experimental groups.
Keywords |
||||
| Ecotoxicology; Skeleton; Herbicides; Reptiles; South American River Turtle | ||||
INTRODUCTION |
||||
| Technological progress enabled the evolution of several areas related to food production, health, and agriculture. However, these improvements are intimately connected with the use of chemical compounds that still have unknown properties (Oga, 2003). Nowadays, pesticides are the most important environmental contaminants from human activities that cause severe problems to living organisms. Given the characteristics of these compounds and their persistence in the biosphere, this kind of pollutant may affect natural communities, causing impacts and deleterious effects at tissue and molecular levels in animals (Bueno-Guimarães et al., 2001; Berti et al., 2009). | ||||
| The use of herbicides in weed control has been recognized all over the world as a useful agricultural practice. However, its indiscriminate use may impact non-target organisms, mainly aquatic organisms (Nwani et al., 2010). Brazil is considered the largest consumer of pesticides in the world (ANDEF, 2012), and herbicides, mainly atrazine and glyphosate, are the most used class of pesticides in the country (Cox, 2001; IBAMA, 2010b). | ||||
| Atrazine (2-chloro-4-ethylamine-6-isopropylamine-striazine) is more commonly used in rural areas, mainly in corn, sorghum, and sugarcane crops. Although it is classified as moderately toxic for aquatic species, this herbicide is one of the most common contaminants detected in streams, rivers, lakes, dams, and underground waters (Battaglin et al., 2003; Scrubner et al., 2005; Battaglin et al., 2008). On the other hand, glyphosate (N-(phosphonomethyl) glycine) may be the most important herbicide ever developed (World Health Organization, 1994). Due to its low persistence in the soil and biodegradability, glyphosate is applied repeatedly in weed control of several crops. As, glyphosate sales represent 76% of the total trade of herbicides in Brazil (IBAMA, 2009), large amounts of this pesticide may reach non-target organisms (Mitchell et al., 1987; Servizi et al., 1987). | ||||
| In 2006, the legal regulations of the herbicide glyphosate started to be reviewed in Brazil. Today, this review request is under analysis, according to the resolution RDC no. 10/2008 (ANVISA, 2014). After eight years of study, the Genetics and Environmental Mutagenesis Research Group (GEMA) made up by researchers from the Universidade Nacional de Río Cuarto (UNRC), issued a report in which they linked the use of glyphosate with genetic changes that may lead to spontaneous miscarriage, fetal malformation, and cancer (Torres et al., 2006). However, the greatest obstacle to this review of glyphosate use is posed by lawsuits from manufacturers of the products that attempt to stop the review process. | ||||
| Wild fauna is exposed to a wide array of conditions and synthetic chemical products in the environment. Ecotoxicological studies have demonstrated that many of these compounds may affect wild animal survival, interfering with the individual functioning of organisms, causing disease, and affecting reproduction (Guillette and Crain, 2000). Although aquatic environments may be contaminated by different pollutants, little is known about the real effects of these compounds, their toxicological properties, and the risks they pose to the fauna, especially to reptiles. Among these animals, the order Testudines has shown to be of great ecotoxicological importance. Representatives of this group are useful indicators of chemical and radioactive contamination because of their wide geographical distribution, variety of habitats, and longevity (Meyersschöne and Walton, 1994). | ||||
| The Amazon aquatic community of Testudines is one of the most diverse in the world (Mittermeier, 1978). The species Podocnemis expansa is one of the main representatives of this order, which is considered the largest freshwater species of Testudines in South America, reaching up to 107 cm in shell length and weighting 90 kg. These animals are subjected to environmental influences (temperature, water, and gas exchanges) that interfere both with their embryonic development and sex determination (Malvasio, 2001; Pough et al., 2008). | ||||
| Due to the use of their eggs and meat (Pearse et al., 2006) by riverside communities, P. expansa population has been drastically reduced (Moll and Moll, 2004), and became regulated by Appendix II of the Convention on International Trade in Endangered Species (CITES). These animals were also listed by the IUCN as a low risk, conservation-dependent species (IUCN 2011). Nowadays, the Centro Nacional de Pesquisa e Conservação de Répteis e Anfíbios, an unit of Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) is responsible for conservation and management projects involving Testudines, with P. expansa as one of the most protected and studied species. | ||||
| Aquatic organisms are successively exposed to a variety of contaminants, mainly from agricultural activities, such a heavy metals, hydrocarbons, organic compounds, and pesticides (Schnurstein and Braunbeck, 2001). Given the scarcity of studies related to the exposure of Testudines, and the possible effects that these products may cause to these animals, the objective of this study was to assess the effects of the herbicides atrazine and glyphosate in bone development of P. expansa embryos exposed to different concentrations of these pesticides during artificial incubation. | ||||
MATERIAL AND METHODS |
||||
Egg collection |
||||
| In October 2013, a total of 140 eggs of P. expansa were collected in an environmental protection area Meandros do Rio Araguaia, Brazil (13º 20' 38" S and 50º 38' 05" W; SISBIO/ ICMBio license no. 36957-1/2012). All procedures in the study were approved by the Research Ethics Committee from Universidade Federal de Uberlândia (CEUA/UFU 055/12). | ||||
| Eggs were removed from the nests, placed in plastic bags with vermiculite moistened with water 2:1 v/v, and sent to the Wild Animal Research and Teaching Laboratory at Universidade Federal de Uberlândia (LAPAS/UFU) for artificial incubation. Artificial incubation and exposure to the pesticides | ||||
| Eggs were artificially incubated in seven trays placed in the incubators according to the method by Verdade et al. (1992), and maintained at 28-31°C and 80-100% relative humidity throughout the incubation. | ||||
| Sand from the site of egg collection was used as the incubation substrate. This substrate was contaminated with atrazine or glyphosate-based commercial herbicides in predetermined concentrations based on the maximum limit of 2 μg/L e 65 μg/L, respectively, for atrazine and glyphosate contamination in superficial waters determined by the Conselho Nacional do Meio Ambiente (CONAMA) resolutions no. 357/2005 and no. 20/1986. Substrates were moistened daily with pure distilled water or distilled water contaminated with either Atrazine PROOF® at concentrations equal to 2, 20 and 200 parts per billion (ppb) or Glyphosate Roundup Original®, at concentrations equal to 65, 650, and 6500 ppb, making up a control group and six experimental groups. A total of 20 eggs of P. expansa were incubated in each group. | ||||
Handling of the embryos |
||||
| Eggs were placed in the incubation trays on Day 0. From this day on, two eggs were collected from each incubator every ten days until hatching. A total of 10 eggs were collected per treatment, in average. After eggs were collected, embryos were killed humanely following specific technical guidelines and regulations. Eggshells were cut with surgical scissors, embryos were removed and immediately received sodium pentobarbital (Close et al., 1997) in a dose greater than 60 mg/Kg by intracoelomic route (Reilly et al., 2001). This procedure ensured overdose by anesthesia, with quick unconsciousness followed by death by respiratory arrest. After animals were euthanized, embryos were preserved in formaldehyde 3.7%. Diaphanization of soft tissues and staining of cartilage and bones. Embryos soft tissues were diaphanized with potassium hydroxide (KOH), and bones and cartilages were stained with Alizarin red S and Alcian blue, respectively, according to the methods by Davis and Gore (1936) and Dingerkus and Uhler (1977), with some modifications. | ||||
Data record and analysis |
||||
| The specimens were analyzed in a stereomicroscope (Leica, DM 1000). Morphological characteristics of the cartilages and bones of the embryos exposed to atrazine were compared with the results obtained by Vieira (2008), who described bone ontogeny in P. expansa embryos. | ||||
RESULTS AND DISCUSSION |
||||
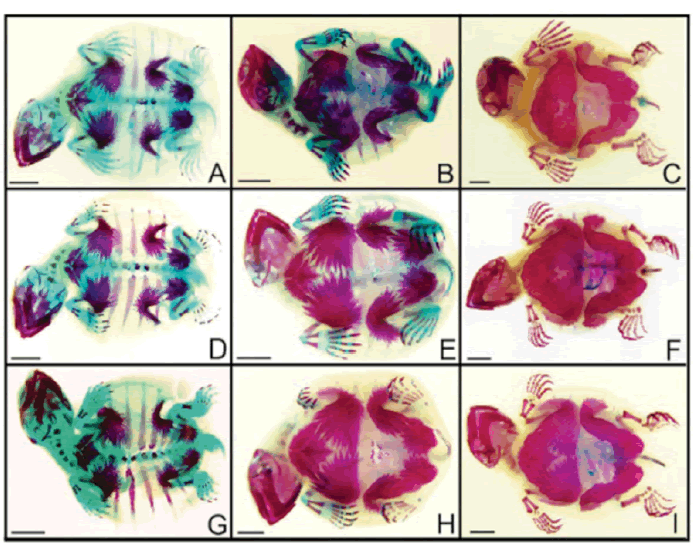
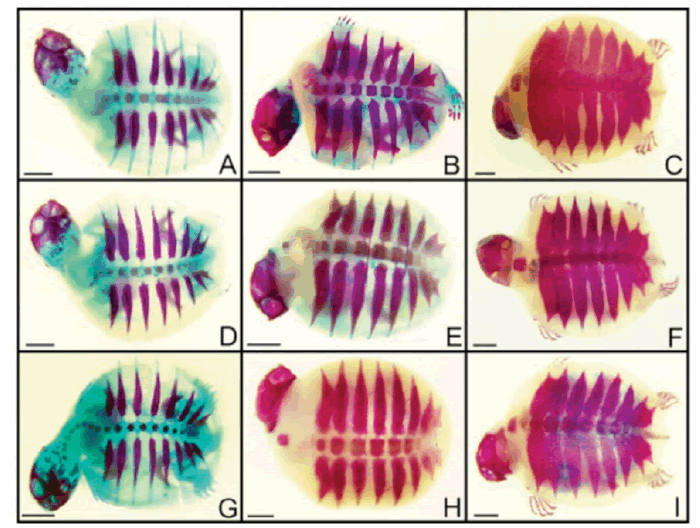
| After tissue analysis by diaphanization and staining, embryos were evaluated for the expected bone development based on Vieira (2008). No changes were observed in bones and cartilages of the embryos exposed to atrazine and glyphosate, in none of the concentrations analyzed. Figure 1shows ventral view of some embryos in different develpment stages exposed to some of the herbicide concentrations analyzed. Dorsal views are shown in Figure 2. | ||||
| As for the herbicides used in the present study, it has to be emphasized that both atrazine and glyphosate have been widely studied, mainly in relation to exposure of living organisms. The absence of adverse effects in animals exposed to these contaminants has also been described in other species. Le Mer et al. (2013) contaminated eggs of a fish species (Gasterosteus aculeatus) in laboratory, during 42 days of incubation, using four concentrations of atrazine and glyphosate (0.1; 1; 10 and 100 μg/L). After they hatched, alevins were evaluated in relation to their mass (length, wet weight), and biochemical and histological analyses were carried out (genotype and phenotype analysis). According to these tests, no significant effect of these pesticides was observed in the initial development phases of these alevins. | ||||
| Studies carried out with reptiles using a glyphosatebased compound, Roundup®, showed a decrease in white cell counts, an increase in heterophile antibodies, changes in plasma proteins, besides a negative effect on the development of juvenile Caiman latirostris alligators (Latorre et al., 2013). In those studies, juvenile individuals were exposed to concentrations of the herbicide equal to 11 and 21 mg/L for two months, and were compared with a control group. The herbicide concentration was progressively decreased throughout the exposure period to simulate glyphosate degradation in water. It should be emphasized that, in this study, different from the present one, animals were directly exposed to the pesticide. | ||||
| Several studies have been carried out on the pesticide toxicity, mainly with amphibians, given the fact that pesticide absorption is quicker in these animals than in other vertebrates (Quaranta et al., 2009). Relyea (2005) sprayed glyphosate directly on the animals using the doses recommended in the USA, and observed that 79% of the population of juvenile frogs and toads died in the first 24 hours of the experiment. Another study with anurans showed that 30% of the animals died (Bernal et al., 2009). However, it is known that not all glyphosate-based herbicides present immediate risk to amphibians in field conditions, as mortality may range from 0% to 80%, depending on the formula (Dinehart et al., 2009). Therefore, similar to what is observed with mortality rates, adverse effects of exposure may vary depending on the formula, including in P. expansa. | ||||
| As for atrazine exposure, Storrs and Semlitsch (2008) demonstrated that as amphibians have a shorter larval period and, consequently, quicker development, they are more susceptible to atrazine contamination. Other authors reported that the exposure to this herbicide may cause hermaphroditism, demasculinization, and gonad malformation in African clawed frogs (Xenopus laevis) (Hayes et al., 2002; Hayes et al., 2003). As for the effects of atrazine in reptiles, there are few reports in the literature. De Solla et al. (2006) observed changes in gonad development with different atrazine concentrations in Chelydra serpentine. Exposure to atrazine during embryonic development of Graptemys ouachitensis and Graptemys pseudogeographica turtles inhibited flight response and reduced post-hatching survival (Neuman-Lee and Janzen, 2011). However, physical aptitude parameters have not been evaluated for P. expansa. | ||||
| Other studies demonstrated physiological and biochemical effects of herbicides on living organisms. Glyphosate and atrazine affected phagocytosis efficiency in immunological cells of fish (Rhamdia quelen), and animals showed greater susceptibility to bacterial infections (Kreutz et al., 2010). After exposure to sublethal concentrations of these pesticides for 96 hours, the authors reported a significant decrease in the number of cells and in intracoelomic phagocytosis rates. These fish were also more susceptible to pathogenic bacteria. | ||||
| Studies on the evaluation of embryo and fetal toxicity, and of the teratogenic potential of atrazine in rats and rabbits showed results that are also different from those of the present study. Atrazine was administered by oral route in doses equal to 0, 10, 70 or 700 mg/kg to groups of 6 to15 day pregnant rats; and in doses equal to 0, 1, 5 or 75 mg/kg to 7 to 19 day pregnant rabbits (Infurna et al., 1988). Maternal and fetal toxicity were observed both in rats and rabbits at the highest concentrations. However, no teratogenic effect was observed in any of the species. | ||||
| In studies on reproductive toxicity in rats, mothers were exposed to 500, 750 or 1000 mg/kg of a glyphosate formula by oral route and animals showed incomplete ossification of cranial bones, increased size of the fontanels, bipartite interparietal and supraoccipital bones, absence of tail vertebrae, undulation in ribs, and absence of metatarsal bones and phalange ossification in pelvic limbs. Changes were observed in all concentrations (Dallegrave, 2003), demonstrating the teratogenic power of glyphosate, in spite of the absence of bone effects in P. expansa, as observed in this study. In these animals, the eggshell may have functioned as a vital protective barrier. | ||||
| The eggshell in Testudines is highly important for the embryo, once it controls gas exchanges through the pores, is a source of minerals for the embryo, and functions as a protective barrier (Kitimasak et al., 2003; Kusuda et al., 2013). In terms of chemical composition, other studies showed that the eggshell in Testudines is an extremely good barrier: only in extreme conditions may external moisture influence embryo development (Lesem and Dmi'el, 1986). Exposure of P. expansa eggs to technical grade atrazine for only one day of artificial incubation caused changes in the chemical composition of the eggshell in terms of phosphorus concentrations, fat content, and thickness. However, no teratogenic effect was observed in bone ontogeny (Souza, 2013). Given the methodology and results of the present study, it may be suggested that the eggshell was an important barrier, preventing herbicides from reaching the interior of the eggs. However, the study was not designed to evaluate the ability of the eggshell to function as a barrier. | ||||
| As for the absence of macroscopic changes in bone ontogeny of P. expansa after exposure to atrazine and glyphosate, the present study showed different results compared with literature reports on other pesticides. In studies with juvenile Alligator mississippiensis females living in Apopka lake in Florida, which is contaminated with DDT, Lind et al. (2004) observed greater trabecular density in long bones in computed tomography, suggesting that bone resorption in these animals was compromised by the inhibition of osteoclastic activity. | ||||
| Studies with malformations in amphibians and fish also reported teratogenic effects. Rana perezi tadpoles kept for 14 weeks in water containing two lethal concentrations of the insecticides ZZ-Aphox (Carbamate) or Folydol (Organophosphate) (0.25 and 1 mg/L, respectively) showed malformations in the spine and/or limbs, besides differences in bone matrix and abnormal vascularization of the periosteum (Alvarez, 1995). Wells and Cowan (1982) showed vertebral dysplasia in fish exposed to the herbicide Trifluralin. In an experiment carried out in fish tanks, the species Salmon parr was exposed to three doses of the contaminant (0.5, 0.25 and 0.01 mg/L) for 16 hours during 10 days. X-ray analysis showed clear signs of vertebral damage in these animals, mainly in those that were exposed to the highest concentrations. In these studies, different from what happened to P. expansa, the animals were in direct contact with pesticides and showed changes in morphology. | ||||
| Bone malformations were also observed in recently hatched chicks. Uggini et al. (2010) injected commercial insecticides at different concentrations in chicken eggs on day zero of incubation. After hatching, live and dead chicks were analyzed. The specimens were stained with Alizarin and Alcian blue for the analysis of the cartilages. Embryo malformations were found in the axial and appendicular skeleton at all concentrations. In the lowest concentration (0.01 μg), the morphologic effects were not obvious, but increasing concentrations showed more evident effects, such as bent legs and distorted phalanges, as well as deformities in the beak, sternum, ribs, and other sites. Other trials with birds corroborated these findings (Rao et al., 1992; Anwar, 2003; Ahmad and Asmatullah, 2007). | ||||
| In spite of the daily contamination using Atrazine PROOF® and Glyphosate Roundup Original®, no changes were observed in P. expansa bone development. However, it cannot not be stated that exposure may not have changed other parameters, such as physiology, neurology, or behavior of the individuals, as observed and discussed by other authors. | ||||
CONCLUSION |
||||
| Exposure to commercial herbicides atrazine and glyphosate in concentrations up to 100 times greater than those allowed by current regulations did not cause any bone abnormality in P. expansa embryos exposed daily to the pesticides during their ontogeny. | ||||
ACKNOWLEDGMENTS |
||||
| We would like to thank RAN/ICMBio and CEUA/UFU for allowing this study to be carried out. We would also like to thank Omar Teodoro da Silva Júnior for providing the herbicides, and to Lilian Freitas Bastos for the aid in egg collection. We also thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial aid and support for the study. | ||||
Figures at a glance |
||||
|
||||
References
|