Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2019) Volume 9, Issue 66
Development and validation of an Enzyme Linked Immunosorbent Assay (ELISA) for gentamicin quantification in dried blood spot samples
Teng Meng1, Abdel Qader Al Bawab1,2, Ahmed Hawwa1, James McElnay1*
1Clinical and Practice Research Group, School of Pharmacy, Queen’s University Belfast, Belfast, United Kingdom
2Faculty of Pharmacy, Al Zaytoonah University of Jordan, Amman, Jordan
- *Corresponding Author:
- James McElnay
Clinical and Practice Research Group School of Pharmacy
Queen’s University United Kingdom
E-mail: j.mcelnay@qub.ac.uk
Accepted Date: February 08, 2019
Citation: Meng T et.al. Development and validation of an Enzyme Linked Immunosorbent Assay (ELISA) for gentamicin quantification in dried blood spot samples. Asian J Biomed Pharmaceut Sci. 2019;9(66):10-15.
DOI: 10.35841/2249-622X.66.18-1176
Visit for more related articles at Asian Journal of Biomedical and Pharmaceutical SciencesAbstract
A competitive enzyme linked immunosorbent assay (ELISA) procedure has been developed for the quantification of gentamicin in dried blood spot (DBS) samples collected from paediatric patients on Guthrie cards. Gentamicin was extracted from DBS samples by vortexing with the ELISA extraction buffer for 30 minutes. The ELISA assay was successfully validated using ICH guidelines for assay validation over the concentration range of 0.15-2.5 μg/ml. The standard deviation, mean, coefficient of variation (CV%) and relative error (RE%) were evaluated and demonstrated good assay reproducibility. The validated ELISA method can be applied to clinical DBS samples. This assay will be utilised to quantify gentamicin in DBS samples collected from paediatric (premature neonatal) patients, in which the quantification data will be utilised for population pharmacokinetic studies of gentamicin in premature neonates.
Keywords
Gentamicin, ELISA, Dried blood spot (DBS) sampling
Introduction
Gentamicin (Figure 1), has been used in neonates since 1970 to treat bacterial infections caused by Gram-negative bacteria [1,2]. In the neonatal intensive care unit (NICU), gentamicin and benzyl penicillin are often prescribed by the intravenous route as the first line treatment of early onset sepsis. Like the other aminoglycosides, gentamicin works by irreversibly binding to the 30S subunit of the bacterial ribosome, thus inhibiting protein synthesis leading to a bactericidal effect. The reported therapeutic range for trough and peak plasma concentrations of gentamicin is below 2 μg/ml and 5-12 μg/ml respectively [3]. It has been traditionally assumed that ototoxicity and nephrotoxicity, well known side effects of gentamicin, are associated with high trough plasma levels of gentamicin [4], i.e. trough levels above 1 to 2 μg/ml [5]. Although frequently prescribed, there is a paucity of information available on clinically appropriate dosing of gentamicin in premature neonates [6].
Conventionally, plasma is the gold standard matrix for the assessment of pharmacokinetic (PK) properties of a medicine in all patient age groups, but due to the comparatively lower circulatory volume in neonates, there is a risk of iatrogenic blood loss with the multiple blood sampling required for PK analysis. Besides, regulatory laws restrict the volume of blood collection for research purposes from such a vulnerable population, which represents a significant challenge to the performance of clinical research within this age group [7].
Thus, the application of a micro-sampling approach is of importance. An already well-established micro-sampling approach to determine medication concentrations in blood samples is dried blood spot (DBS) sampling, which typically requires only 15-20 μl of whole blood per sample. Dried blood spot sampling involves the collection of blood samples via a heel prick, a finger prick (older children and adults) or via an indwelling catheter if one is already in place. The sample is collected on an absorbent paper card e.g. Guthrie card, allowed to dry and then stored until analysis [8]. This minimally invasive and simple approach is particularly suitable for collecting samples in patients at the extremes of age such as in infants and neonates, and in the elderly [9,10]. The potential for the use of DBS samples in therapeutic monitoring and pharmacokinetic studies has been promoted by a range of researchers via linking this sampling method with high performance liquid chromatography (HPLC) with UV or mass spectrometry detection [8,11-14].
Many quantitative methods had been described to analyse gentamicin in serum or plasma including HPLC coupled with mass spectrometry [15], thin-layer chromatography [16], HPLC with charged aerosol detection [17] and HPLC combined with pulsed electrochemical detection [18]. In clinical practice, however, enzyme-linked immunosorbent assay (ELISA) is the most frequently used analytical platform for the analysis of gentamicin. ELISA is a technique that utilises antibodies and a colour change to identify and quantify the analyte of interest [19]. Competitive ELISA which is a competitive enzyme immunoassay has high specificity and sensitivity for the quantitative analysis of gentamicin mostly in, but not limited to foodstuff, serum and urine. In the present research, the DBS sampling and competitive ELISA platforms were combined to form a new methodological approach for the clinical quantification of gentamicin for use in pharmacokinetic studies. This is the first report describing the quantification of gentamicin in the DBS matrix.
Materials and Methods
Materials and equipment
Gentamicin sulphate was purchased from Sigma-Aldrich (Irvine, UK). MaxSignal®.Gentamicin ELISA test kits were purchased from Bioo Scientific (Catalogue No.:1027-01; Kennett, UK). Deionised water was obtained using a Purelab Maxima purification system from ELGA (Marlow, UK). Guthrie cards (3M-226) were purchased from 3M Security Systems Division (Oldham, England) and were used for sample collection. Blank blood for assay development was collected from healthy human volunteer subjects in concordance with a procedure approved by the Ethics Committee in the School of Pharmacy, Queen’s University Belfast.
A single 8 mm punch, to punch the DBS samples from the Guthrie cards was obtained from Darice Inc. (Strongsville, USA). A Stuart® SB2 rotator supplied by Sigma-Aldrich (Irvine, UK) was used to prepare blood samples containing gentamicin. A PowerWave™ XS Microplate Reader and KCJr software from Bio-Tec (Winooski, USA) was utilised in the analytical quantification of gentamicin.
Sample preparation
Preparation of stock solution and working standards: Gentamicin sulphate (10 mg), which is equivalent to 6.5 mg free base, was dissolved in 65 mL deionised water to obtain a 100 μg/ml stock solution of gentamicin. The stock solution was stored at -80 °C until required. Seven working standard solutions at concentration levels of 3, 5, 10, 15, 20, 40, 50 μg/ml were prepared by further dilution with deionised water. Four of these seven concentrations were also used as quality control (QC) analytes (3, 5, 15 and 50 μg/ml), while all seven calibrators were used for the development of calibration curves.
Preparation of calibration standards and quality control (QC) samples: Gentamicin calibration solutions (50 μl) of different concentrations were added into 950μl aliquots of fresh blood followed by 45 minutes of soft-mixing using a StuartSB2 fixed speed rotator with tube holder to acquire final blood concentrations of 0.15, 0.25, 0.5, 0.75, 1, 2, 2.5 μg/ml. The assigned low, middle and high QC concentrations were 0.25, 0.75 and 2.5 μg/ml respectively, and drug free blood (without gentamicin) was used as a blank.
Blood spotting
Samples of spiked whole blood (15 μl) were spotted onto Guthrie cards to provide a range of DBS samples of known concentration. The samples were air-dried overnight at room temperature. During this period, the samples were not allowed to come into contact with direct sunlight or heat (kept in a cupboard at ambient temperature). Once dried, the samples were individually placed into plastic Ziploc bags, labelled and stored in a sealed polypropylene container with desiccant (silica gel) in a freezer (-80) until required for analysis.
Extraction of dried blood spot (DBS) samples for analysis
The complete DBS samples (15 μl) were punched from Guthrie cards using an 8 mm diameter punch, placed in a clean 2 ml Eppendorf tube and extracted using 735 μl of the ‘Sample Extraction Buffer’ provided in the test kit (MaxSignal). The samples were vortexed for 30 minutes to allow sufficient time for full extraction. The tubes were then centrifuged at 20,000 g for 10 minutes using a SIGMA 2-16K Centrifuge to obtain the supernatant for analysis.
Competitive Enzyme-linked Immunosorbent Assay
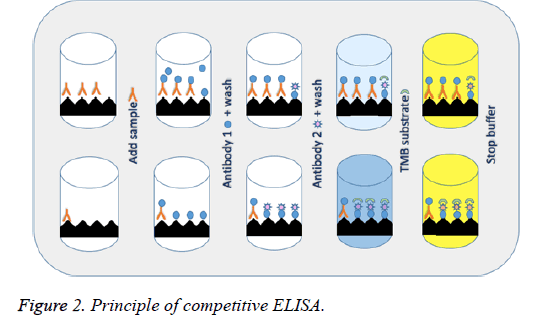
Competitive ELISA is typically performed on a 96-well plate which is coated with an antigen (the drug of interest; in this case gentamicin). Samples to be analysed are then added along with a primary antibody (specific for the target of interest) to the micro-wells. If gentamicin exists in the sample, it will compete for the antibody and prevent the primary antibodies from binding to the drug attached to the well. The secondary antibody targets the primary antibody that is complexed to gentamicin coated on the micro-wells. After addition of the substrate, the enzyme produces a signal (colour intensity) which has an inverse relationship to the sample concentration, i.e. the higher the concentration the weaker the signal (lighter the colour) (Figure 2).
50 μl of the DBS sample extracts from the top of the supernatant were withdrawn and added in duplicate to 96-wells plates in each analytical run, followed by the addition of 100 μl of the primary antibody solution and mixed well by gently shaking the plate manually for one minute. The mixtures were incubated for exactly 30 minutes at room temperature. Horseradish Peroxidase (HRP)-conjugated secondary antibody solution was then added with gentle mixing, and incubated for a further 30 minutes at room temperature. The reaction was visualized by the addition of 100 μl a chromogenic substrate (tetramethylbenzidine; TMB) and further incubation for 15 minutes. The plates were washed three times with washing buffer after each step. After the final incubation step, the reaction was stopped with 100 μl stop buffer and absorbance at 450 nm was measured using a PowerWaveTM XS plate reader. The absorbance values obtained from the KCJr software were exported into an Excel sheet ahead of data analysis. Because the kit is not certified for whole blood analysis, quantitative method validation of the new biomatrix (whole blood) was necessary.
Validation procedures
All validation experiments in DBS samples were performed in accordance with current recommendations for bioanalytical methods according to the International Conference of Harmonisation (ICH) guidelines for HPLC method validation [20].
Linearity
The linearity of the method was established by plotting the mean relative absorbance values for the seven calibration concentrations on three consecutive days. In order to achieve the best fit for the calibration curves, a semi-logarithmic scale was used. The y-intercept, slope and correlation coefficient were determined.
Precision and accuracy
Precision and accuracy were accomplished by analysing QC samples prepared at three concentration levels (0.25, 0.75, 2.5 μg/ml) and the lower limit of quantification (LLOQ) sample (0.15 μg/ml). Three replicates of each level were assayed in one run for the within-day determinations. The same levels of QC and LLOQ concentrations were also assayed on three consecutive days to provide the between-day accuracy and precision data. The precision of the method was expressed as percentage coefficient of variation (CV%). The accuracy was expressed as percentage relative error (RE%). An accepted deviation of back-calculated concentrations from the best fitted regression line should be within 15% for all points except for the lower limit of quantification which should be within 20% [20].
Lower limit of quantification
On the basis of the standard curve, the LLOQ of the samples was the lowest concentration in the curve at which CV% and RE% were less than 20%, i.e. the allowed limit according to the ICH guidelines.
Results
Assay development
Four different standard solutions at concentration levels of 1.5, 3, 15, 50 μg/ml were obtained by diluting the stock solution. DBS samples were prepared as mentioned above to achieve final blood concentrations of 0.075, 0.15, 0.75, 2.5 μg/ml. During the extraction process, the DBS samples were diluted 50-fold with ‘Sample Extraction Buffer’ to give concentrations of 1.5, 3, 15, 50 ng/ml. Relative absorbance for whole blood samples in the form of dried blood spots and for gentamicin standards supplied with the commercial ELISA kit were compared (Table 1). The mean and standard deviation of the difference between the two matched readings were calculated to determine the equivalence between the two methods. These promising preliminary results indicated that whole blood DBS samples can be combined with ELISA for quantitative analysis of gentamicin.
| Concentration of gentamicin (ng/ml) | Relative absorbance | Mean absorbance | Standard Deviation (SD) | |
|---|---|---|---|---|
| Gentamicin standard | Blood samples | |||
| 50 | 0.3 | 0.37 | 0.34 | 0.05 |
| 15 | 0.6 | 0.73 | 0.67 | 0.09 |
| 3 | 1.18 | 1.4 | 1.29 | 0.16 |
| 1.5 | 1.37 | 1.62 | 1.5 | 0.18 |
Table 1. Comparison of relative absorbance for blood samples and gentamicin standards (provided in ELISA kit).
Assay validation
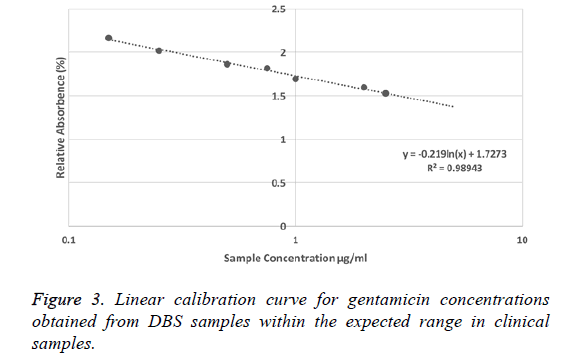
Linearity: A typical semi-log calibration curve using the full range of gentamicin concentrations in DBS samples within the expected concentration range for clinical trough levels of the drug was constructed (Figure 3). The mean slope and intercept.
Precision values from the representative calibration curve were -0.219 and 1.727 respectively and accuracy: Following the ICH guidelines, the within and between-day accuracy and precision values for gentamicin assay were estimated using the data from the three-day validation work at four QC levels (0.15, 0.25, 0.75, 2.5 μg/ml). The results for the accuracy and precision measurements are presented in Table 2. Within and betweenday precision and accuracy at QC levels were adequate with CV% and RE% values falling within the allowed range (lower than 15% for QC samples and lower than 20% for the LLOQ samples).
| Nominal concentration (ug/ml) | Precision | Accuracy | |
|---|---|---|---|
| Mean ± SD | CV% | RE% | |
| Intra-day | |||
| 2.5 (HQC) | 2.44 ± 0.31 | 12.52 | 2.56 |
| 0.75 (MQC) | 0.78 ± 0.03 | 3.64 | -3.78 |
| 0.25 (LQC) | 0.27 ± 0.04 | 14.34 | -6 |
| 0.15 (LLOQ) | 0.17 ± 0.02 | 12.43 | -14.67 |
| Inter-day | |||
| 2.5 (HQC) | 2.31 ± 0.17 | 7.2 | 7.47 |
| 0.75 (MQC) | 0.77 ± 0.06 | 8.08 | -2.44 |
| 0.25 (LQC) | 0.22 ± 0.02 | 9.42 | -10.53 |
| 0.15 (LLOQ) | 0.16 ± 0.01 | 8.97 | -5.77 |
Table 2. The intra-day and inter-day validation of gentamicin determination in DBS samples using ELISA methodology.
Lower limit of quantification: The lower limit of quantification was determined to be 0.15 μg/ml in whole blood (within a DBS sample) in this assay. Both accuracy and precision were within the allowed limits at this concentration point.
Discussion
Gentamicin has proven efficiency against Gram-negative infections; it demonstrates a concentration dependent bactericidal effect and a post-antibiotic effect, which can lead to the continuous bactericidal effect when blood levels drop below the minimum inhibitory concentration [21,22]. It is associated with low levels of resistance in common nosocomial pathogens, primarily Enterococcus [23,24]. Studies of gentamicin-associated toxicity are less robust in neonates, as hearing loss and renal impairment after severe bacterial infection are often multifactorial in origin [25], however, it is expected that trough levels above 1 to 2 μg/mL are likely to be associated with toxicity.
Competitive ELISA has high specificity and a higher sensitivity than other approaches for the analysis of gentamicin. The method used in the present work has been validated for the first time using DBS samples. The methodology exhibited appropriate linearity, accuracy and precision. The drawback of the ELISA assay developed in the present report is that the linear calibration range could only be achieved over a relatively narrow range of gentamicin concentration within a single experimental run (in our case between 0.15 and 2.5 μg/mL) for gentamicin.
Conclusion
For the first time, the technique of DBS sampling coupled with ELISA quantitative analysis was shown to be a very promising approach for gentamicin bioanalytical quantification, for example, in gentamicin pharmacokinetic studies. These results overall suggest that the ELISA-DBS method developed for analysing expected trough concentrations in neonates, could be used as a relatively non-invasive sampling approach when compared to venepuncture sampling in the clinical setting. If samples are found to exceed the upper limit of the calibration curve, extracts can be diluted by a factor of ten times and reanalysed. The validated assay has been utilised to quantify trough steady state blood concentrations of gentamicin in DBS samples from hospitalised neonates as part of a separate study.
Acknowledgement
Thanks to everyone who helped us with this study. We are very grateful to the Deputy of Research of Hormozgan University of Medical Sciences. Thank you very much from the Student Research Committee of Hormozgan University of Medical Sciences.
Funding
This paper presents independent research carried out as part of routine work of authors.
Availability of Data and Materials
The datasets used and/or analysed during the current study are available on reasonable request. All data generated or analysed during this study are included in this published article.
Authors’ Contributions
Teng Meng analyzed the volunteer’s blood samples regarding the validation of the analytical method and the quantification of gentamicin and wrote the manuscript. Abdel Qader Al Bawab interpreted the data and was a contributor in reviewing the manuscript. Ahmed Hawwa was a contributor in supervising the experimental procedure. Professor James McElnay was the correspondence author. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
This study was approved by the Ethical Committee of Hormozgan University of Medical Sciences.
Competing Interests
The authors declare that they have no competing interests in the current research.
References
- Young TE. Aminoglycoside Therapy in Neonates: with Particular Reference to Gentamicin. Neorev. 2002;3:12.
- Moulds RFW, Jeyasingham MS. Gentamicin: a Great Way to Start. Austral Prescr. 2010;33:134-135.
- Darmstadt GL, Miller-Bell M, Batra M, et al. Extended-interval dosing of gentamicin for treatment of neonatal sepsis in developed and developing countries. J Health Populat Nutrit. 2008;26:163-182.
- Chattopadhyay B. Newborns and gentamicin-how much and how often? J Antimicrob Chemother. 2002;49:13-16.
- Mohamed AF, Nielsen EI, Cars O, et al. Pharmacokinetic-pharmacodynamic model for gentamicin and its adaptive resistance with predictions of dosing schedules in newborn infants. Antimicrob Agents Chemother. 2012;56:179-188.
- Ahmed U, Spyridis N, Wong IC, et al. Dosing of oral penicillins in children: is big child-half an adult, small child-half a big child, baby-half a small child still the best we can do? British Medi J. 2011;343:7803.
- Weiss M, Fischer J, Boeckmann M, et al. Eliminating discard volumes in neonatal and pediatric blood sampling from arterial catheters: A comparison of three simple blood-conserving aspiration techniques. Pediat Crit Care Medi. 2002;3:134-140.
- Yakkundi S, Millership J, Collier P, et al. Development and validation of a dried blood spot LC-MS/MS assay to quantify ranitidine in paediatric samples. J Pharma Biomed Anal. 2011;56:1057-1063.
- McDade TW, Williams S, Snodgrass JJ. What a Drop Can Do: Dried Blood Spots as a Minimally Invasive Method for Integrating Biomarkers into Population-Based Research. Demograp. 2007;44:899-925.
- Lakshmy R. Analysis of the use of dried blood spot measurements in disease screening. J Diabet Sci Tech. 2008;2:242-243.
- Aburuz S, Millership JS, McElnay JC. Dried blood spot liquid chromatography assay for therapeutic drug monitoring of metformin. J Chromat. 2006;832:2002-2007.
- Deglon J, Thomas A, Daali Y, et al. Automated system for on-line desorption of dried blood spots applied to LC/MS/MS pharmacokinetic study of flurbiprofen and its metabolite. J Pharmac Biomed Analy. 2011;54:359-367.
- Shah NM, Hawwa AF, Millership JS, et al. A simple bioanalytical method for the quantification of antiepileptic drugs in dried blood spots. J Chromatograp Analy Tech Biomed Life Sci. 2013;923:65-73.
- Suyagh M, Iheagwaram G, Kole PL, et al. Development and validation of a dried blood spot–HPLC assay for the determination of metronidazole in neonatal whole blood samples Analyt Bioanaly Chem. 2010;397:687-693.
- Vučićević-Prčetić K, Cservenák R, Radulović N. Development and validation of liquid chromatography tandem mass spectrometry methods for the determination of gentamicin, lincomycin, and spectinomycin in the presence of their impurities in pharmaceutical formulations. J Pharmaceut Biomed Analy. 2011;56:736-742.
- Hubicka U, Krzek J, Woltyńska H, et al. Simultaneous identification and quantitative determination of selected aminoglycoside antibiotics by thin-layer chromatography and densitometry. J AOAC Int. 2009;92:1068-1075.
- Joseph A, Rustum A. Development and validation of a RP-HPLC method for the determination of gentamicin sulfate and its related substances in a pharmaceutical cream using a short pentafluorophenyl column and a charged aerosol detector. J Pharmaceu Biomed Analysis. 2010;51:521-531.
- Li B, Schepdael AV, Hoogmartens J, et al.Mass spectrometric characterization of gentamicin components separated by the new European Pharmacopoeia method. J Pharmaceu Biomed Analysis. 2011;55:78-84.
- Charles PD. ELISA Tests. 2017.
- ICH. International Conference on Harmonisation, Expert Working Group ICH harmonised tripartite guideline Q2 (R1): Validation of analytical procedures: Text and Methodology. 2005.
- Nielsen E, Sandstrom M, Honore P, et al. Developmental Pharmacokinetics of Gentamicin in Preterm and Term Neonates: Population Modelling of a Prospective Study. Clinic Pharmacok. 2009;48:253-263.
- Ponnuthurai J, Varughese N, Laframboise C, et al. An Evaluation of a Gentamicin Dosing Protocol in the Treatment of Bacterial Infections in Neonates. 2016.
- Poole K. Aminoglycoside Resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;2:479-487.
- Chakraborty A, Sarkar S, Gupta MS, et al. Antibiotic resistance pattern of Enterococci isolates from nosocomial infections in a tertiary care hospital in Eastern India. J Natural Sci Biol Med. 2015;6:394-397.
- Musiime GM, Seale AC, Moxon SG, et al. Risk of gentamicin toxicity in neonates treated for possible severe bacterial infection in low- and middle-income countries: Systematic Review. Trop Med Int Health. 2015;20:1593-1606.