- International Journal of Pure and Applied Zoology (2016) Volume 4, Issue 2
Appraisal of Lead In the Organs and Tissues of Domestic Chicken (Gallus Gallus Domesticus) in Ibadan
- *Corresponding Author:
- Emmanuel Teryila Tyokumbur
Department of Zoology, University of Ibadan, Ibadan, Oyo Nigeria, Nigeria
E-mail: e.tyokumbur@mail.ui.edu.ng
Received 18th April 2016; Accepted 24th May 2016; Published 05th June 2016
Abstract
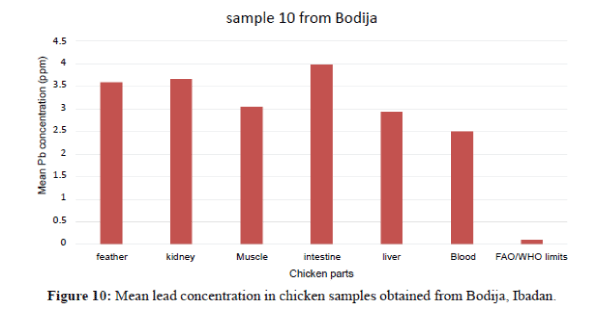
A study was carried out on the assessment of lead in the organs and tissues of domestic chicken (Gallus gallus domesticus) in Ibadan from August to October 2015. Ten (10) chickens (layers and broilers) expressed as samples 1-10 were purchased from different retailer markets (Bodija, Ojoo and Sango) within Ibadan City. The chickens were dissected to remove the intestine, liver, kidney, blood, feathers and muscles were oven-dried at 220°C. The pulverized organ and tissues samples were aciddigested and analyzed for the heavy metal lead (Pb) using Buck Scientific Atomic Absorption Spectrophotometer (AAS). The results showed that the highest Pb concentrations in parts per million (ppm) were recorded in the liver (2.940 ± 0.040), intestine (3.9800 ± 0.500), kidney (3.6600 ± 0.6000), feather (3.5900 ± 0.06000), and muscle (3.400 ± 0.4000) in sample 10, while the lowest concentration was recorded in the kidney (0.150 ± 0.0300) in sample 1 all at Bodija Market. Analysis of Variance (ANOVA) revealed significance of the Pb metal in the organs and tissues of chickens at P<0.05. Less than half of the samples had Pb concentration that exceeded the permissible limit of 0.1 ppm set by FAO/WHO. The study shows that meat from the chicken organs and tissues in Ibadan are relatively safe for human consumption.
Keywords
Lead, Domestic chicken, Blood, Kidney, Liver, Intestine, Muscle, Feather
Introduction
As a result of rapid industrialization and urbanization in developing countries of West Africa, heavy metal pollution is likely to reach disturbing levels. The problem is that preparations are not being made towards the protection of the environment. Nigeria is one of the petroleum exporting countries and use of automobiles by middle and high income groups is rather high. In fact, the growing rate of industrialization is gradually leading to contamination and deterioration of the environment, thus industrialization and heavy metal pollution are positively correlated (Walsh 2000; Olaifa et al. 2004). The Nigerian petroleum contains>0.45 g/l lead as per the Nigerian Industrial Standards (NIS 116:1981). In Nigeria, gasoline with an average lead content of 0.66 g/L remains in use. With high automobile importation, the national consumption of petrol in the country is estimated at 20 million liters per day, with about 150 people/car/city, therefore close to 15,000 kg of lead is emitted into the environment through combustion daily. Heavy metal pollution is posing a serious problem in Nigeria, threatening animals, human health and quality of the environment (Dekofehiniti 2012). Food safety is a major public concern worldwide. The risk of heavy metal contamination in meat is of great concern for both food safety and human health because of the toxic nature of these metals at relatively minute concentrations (Akan et al. 2010). The poultry industry is one of the largest and fastest growing agro based industries in the world. This can be attributed to an increasing demand for poultry meat and egg product (Burel and Valat 2009). Several millions of chickens are raised annually as a source of food for both their meat and eggs. A complete and balanced diet is necessary for human health and vitality. Chicken meat is a major source of protein to man and is widely consumed in Ibadan city and other parts of the world so its safety cannot be ignored. Meat of chicken is rich in many of the essential nutrients including protein (essential amino acids), minerals (e.g., iron, zinc, selenium), vitamins (e.g., Vitamin E) and fat (essential fatty acids such as Omega-3 fatty acids) (Schonfeldt and Gibson 2008). Birds population are particularly susceptible to the effects of anthropogenic activities on the environment. Several biological and physiological processes, such as eating habits, growth, age, breeding, moulting may influence metal concentration and distribution in birds (Kim et al. 2007). The concentration of heavy metals in internal tissues of chicken has been extensively determined by several researchers (Demirbas 1999; Mariam et al. 2004; Iwegbue et al. 2008; Uluozlu et al. 2009). According to Duruibe et al. (2007), some heavy metal ions that are known to be potentially toxic includes aluminium, arsenic, cadmium and lead. Toxic elements can be harmful to birds even at low concentrations when ingested over a long period of time (Young 2005). In recent times, there has been considerable interest in the level of heavy metallic elements in foods because of their deleterious effect on human health (Uche 2012). Apart from those communities exposed to high levels of pollution by industrial effluents or emissions rich in heavy metals, it is evident that, for most individuals food and diet are the most common sources of these potentially toxic heavy metals. These heavy metals in food and drinking water amount to approximately 80% for cadmium, 40% for lead and 8% for mercury (Bennet 1984). Metal contamination in food, especially in meat has been broadly investigated (Sharif et al 2005; Miranda 2005; Lawal et al. 2006; Jukna, 2006). The main objective of the study includes the assessment of the concentration of lead (Pb) in chicken organs widely consumed in Ibadan City, Nigeria and to compare with international guideline standards.
Materials and Methods
Study area
This study was undertaken in Ibadan city which is located near the forest-grassland boundary of South-western Nigeria at a distance some 145 kilometers north east of Lagos. It is the capital of Oyo-State of Nigeria. It has an estimated population of 3.5 million from the projections. The total area of the city is approximately as 103.8 Km2. The Ibadan Region is made up of 11 Local Government areas. It is highly industrialized. Ten (10) live chickens (Gallus gallus domesticus) were collected randomly from different retailer markets (Bodija, Ojoo and Sango) in Ibadan City. A total of 60 samples (muscle, liver, kidney, intestine, blood, and feathers) were used in the study. Sample collection and preparation were carried out between the period of 12th–17th August, 2015.
Samples were identified as 1 to 10, obtained from three different retailer markets in Ibadan namely; Bodija, Ojoo and Sango as shown in Table 1.
| Samples | Location/ source | Date collected |
|---|---|---|
| 1,2, | Bodija | 12/08/2015 |
| 3,4 | Ojoo | 13/08/2015 |
| 5,6,7 | Sango | 14/08/2015 |
| 8,9,10 | Bodija | 17/08/2015 |
Table 1: Location of the samples and dates of collection.
Sample preparation and analyses
The chickens were slaughtered using a dissecting knife. Blood samples were collected and left to coagulate for further analysis. Feathers were collected from each bird. Organs such as kidney, liver, intestine and muscle were separately dissected from the body of each bird. A sizeable portion of each sample was dried in an oven at 220°C for 4 hours until a constant weight was maintained. The dried samples were pulverized using mortar and pestle into powder form and kept in a sample bottle prior to wet digestion. 1 g of each dried sample was digested with 70% nitric acid. 30% hydrogen peroxide was added to the each digested sample in the ratio 2:1 and allowed to simmer down. The digested samples were left overnight at room temperature overnight. Following the digestion, the solution was filtered through a acid resistant filter paper into acid- leached polyethylene bottle. The digestates were made up with 10 ml distilled water and taken for analysis. The concentrations of lead (Pb) were determined using Buck Scientific Atomic Absorption Spectrophotometer (Model 210/ 211 VGP). Each analysis was carried out in triplicate while standard and blank samples were analyzed for every 60 samples.
Data analyses
The data were presented as Mean ± Standard deviation. One way analysis of variance (ANOVA) will be carried on the data to assess any significant difference (p<0.05) in the studied metal between the organs of the chickens, followed by Duncan’s test as a post- hoc test using IBM SPSS Statistics 22.0 software package. The charts will be plotted showing the lead (Pb) concentration in the organs and tissues of Gallus gallus domesticus by Microsoft Excel 2013 software.
Results
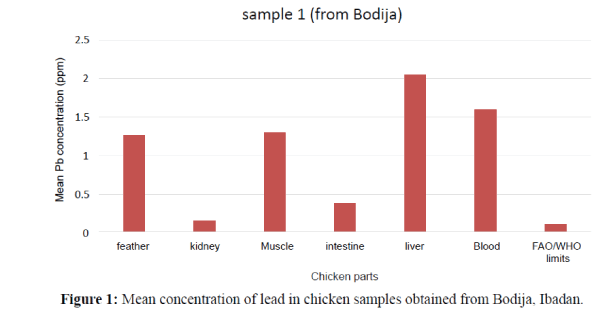
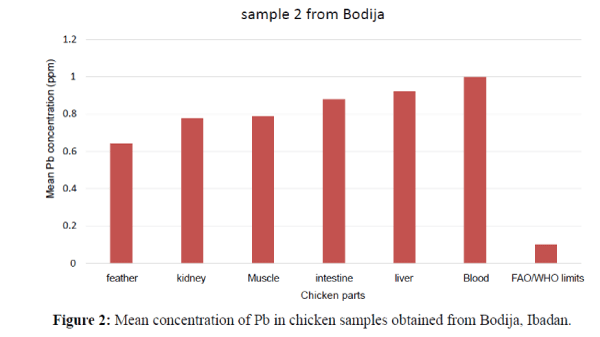
The obtained results are expressed as ppm which revealed the contamination of some chicken organs by the studied element (Pb). The minimum and maximum estimated concentrations of Pb were widely variable in the livers, feather, muscle, and intestine and blood samples. The lead (Pb) concentrations in the study of liver, kidney, muscle, feather, intestine and blood of chicken (Gallus gallus domesticus) has been summarized in Tables 2a, 2b and 3 (Duncan Tests) as shown below. The order of the metal increase in the internal organs and tissues were; blood>feather>muscle>kidney>liver>intestine. In Table 3 the concentrations of Pb metal in the kidney, liver, blood, intestine, feather and muscle of the chickens which were found to be statistically significant at p<0.05. Mean levels with the same alphabet were not significant at (P<0.05) as shown in Table 3. Statistical analysis of different organs and tissue of all the experimental chicken showed that Pb concentrations were significantly higher at (P<0.05) (Figure 1). The liver has the highest concentration as compared to other organs and tissues followed by blood then intestine. The order of Pb increase was: kidney<intestine<muscle< feather<blood<liver. The concentration of Pb in the liver showed significant variation. They exceeded the permissible limit (0.1 ppm) set by FAO/WHO (2002) (Figures 1 and 2). The Pb levels in the kidney and intestine are within the permissible limits in the figure below. Therefore, the kidney and intestine were considered safe for human consumption than other internal organs. The tissues and organs of sample 2 obtained from Bodija showed significant variation. Blood have the highest concentration as compared to other organs. The order of Pb increase was: feather<kidney<muscle<intest ine<liver<blood. They were all above the FAO/WHO (2002) limits (0.1 ppm) as shown below (Figure 3). Therefore, it is unsafe for human consumption.
| Samples | Mean ± SD Min-Max |
Blood | Feather | Kidney | Liver | Muscle | Intestine |
| Sample 1 | 1.597 ± 0.015 1.58 – 1.61 |
1.270 ± 0.020 1.25 – 1.29 |
0.150 ± 0.030 0.12 – 0.18 |
2.050± 0.060 1.99- 2.11 |
1.300 ± 0.040 1.26 – 1.34 |
0.390 ± 0.040 0.35 – 0.43 |
|
| Sample 2 | Mean ± SD Min-Max |
1.000 ± 0.070 0.93 – 1.07 |
0.640 ± 0.050 0.59 – 0.69 |
0.780 ± 0.040 0.74 – 0.82 |
0.920 ± 0.500 0.87 – 0.97 |
0.790 ± 0.050 0.74 – 0.84 |
0.880 ± 0.600 0.82 – 0.94 |
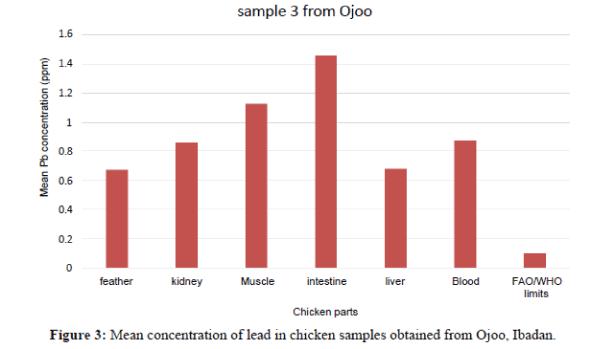
| Sample 3 | Mean ± SD Min-Max |
0.870 ± 0.700 0.80 – 0.94 |
0.670 ± 0.300 0.64 – 0.70 |
0.860 ± 0.800 0.78 – 94 |
0.680 ± 0.500 0.63 – 0.73 |
1.130 ± 0.500 1.08 – 1.18 |
1.460 ± 0.600 1.40 – 1.52 |
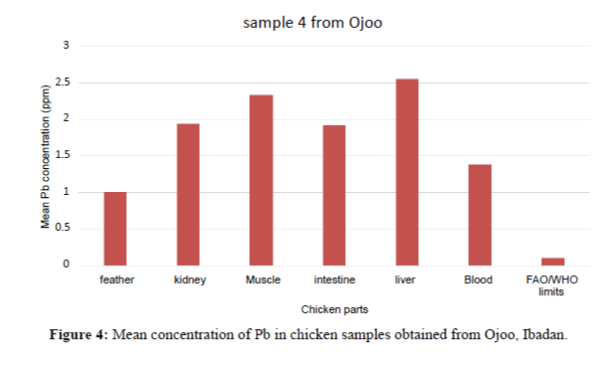
| Sample 4 | Mean ± SD Min-Max |
1.380 ± 0.400 1.34 – 1.42 |
1.000 ± 0.050 0.95 – 1.05 |
1.950 ± 0.020 1.93- 1.97 |
2.550 ± 0.050 2.50 – 2.60 |
2.340 ± 0.300 2.31 – 2.37 |
1.920 ± 0.050 1.87 – 1.97 |
| Sample 5 | Mean ± SD Min-Max |
0.790 ± 0.400 0.75 – 0.83 |
0.840 ± 0.030 0.81 – 0.87 |
1.170 ± 0.040 1.13 – 1.21 |
0.940 ± 0.010 0.3 – 0.95 |
0.890 ± 0.10 0.88 – 0.90 |
0.580 ± 0.020 0.56 – 0.60 |
| Sample 6 | Mean ± SD Min-Max |
2.070 ± 0.070 2.00 – 2.14 |
1.400 ± 0.200 1.38 – 1.42 |
0.640 ± 0.030 0.61 – 0.67 |
0.400 ± 0.030 0.37 – 0.43 |
0.260 ± 0.010 0.25 – 0.27 |
1.020 ± 0.050 0.97 – 1.07 |
| Sample 7 | Mean ± SD Min-Max |
1.960 ± 0.030 1.93 – 1.99 |
1.620 ± 0.036 1.59 – 1.66 |
1.270 ± 0.020 1.25 – 1.29 |
1.430 ± 0.400 1.39 – 1.47 |
1.430 ± 0.050 1.38– 1.48 |
1.690 ± 0.050 1.64 – 1.74 |
Table 2a: Mean concentration of Pb in the organs and tissues of the chicken samples in Ibadan (in ppm).
| Sample | Statistical measure | Blood | Feather | Kidney | Liver | Muscle | Intestine |
| Sample 8 | Mean ± SD Min-Max |
0.330 ± 0.500 0.28 – 0.38 |
1.760 ± 0.070 1.69 – 1.83 |
0.710 ± 0.010 0.70 – 0.72 |
0.520±0.030 0.49 – 0.55 |
1.210±0.050 1.16 – 1.26 |
1.080 ± 0.300 1.05 – 1.11 |
| Sample 9 | Mean ± SD Min-Max |
1.560 ± 0.20 1.54 – 1.58 |
3.310 ± 0.070 3.24 – 3.38 |
2.190 ± 0.050 2.14 – 2.24 |
2.800 ± 0.080 2.72 – 2.88 |
2.630 ± 0.070 2.56 – 2.70 |
2.890 ± 0.080 2.81 – 2.97 |
| Sample 10 | Mean ± SD Min-Max |
2.500 ± 0.050 2.45 – 2.55 |
3.590 ± 0.060 3.53 – 3.65 |
3.660 ± 0.600 3.60 – 3.72 |
2.940 ± 0.040 2.90 – 2.98 |
3.400 ± 0.040 3.00 – 3.08 |
3.980 ± 0.500
3.93 – 4.03 |
Table 2b: Mean concentration of Pb in the organs and tissues of the chicken samples in Ibadan (in ppm).
| Organ/Tissue | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | Sample 7 | Sample 8 | Sample 9 | Sample 10 |
| Feather | 1.270a | 0.6400a | 0.6700a | 1.0000a | 0.8400c | 1.4000e | 1.6200c | 1.7600f | 3.3100e | 3.5900d |
| Kidney | 0.150b | 0.7800b | 0.8600b | 1.9500c | 1.1700f | 0.6400c | 1.2700a | 1.7100c | 2.1900b | 3.6600d |
| Muscle | 1.300c | 0.7900b | 1.1300d | 2.3400d | 0.8900d | 0.2600a | 1.4300b | 1.2100e | 2.6300c | 3.0400c |
| Intestine | 0.390c | 0.880b | 1.4600e | 1.9200c | 0.5800a | 1.0200d | 1.6900d | 1.0800d | 2.8900d | 3.9800e |
| Liver | 2.0500d | 0.9200d | 0.6800a | 2.5500e | 0.9400e | 0.4000b | 1.4300b | 0.5200b | 2.8000d | 2.9400b |
| Blood | 1.597e | 1.0000e | 0.8700b | 1.3800b | 0.7900b | 2.0700f | 1.9600e | 0.3300a | 1.5600a | 2.500a |
Means with the same alphabets were not significantly different from each other at P<0.05.
Table 3: Duncan’s Test of Pb concentration at p<0.05
In Figure 4, the Pb concentration in the organs and tissues of the chicken showed significant variation. The intestines have the highest concentration. The order of Pb increase was: feather<liver<kidney<blood<muscle<intestine. They are above the FAO/WHO permissible limit (0.1 ppm).
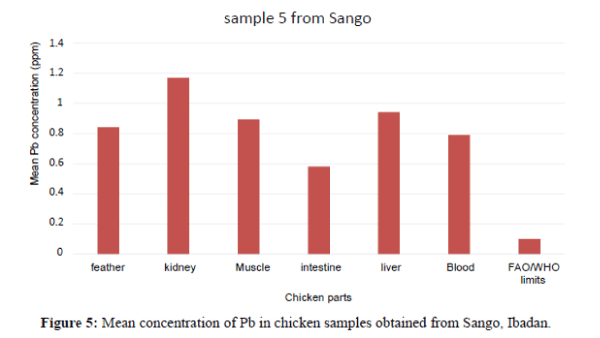
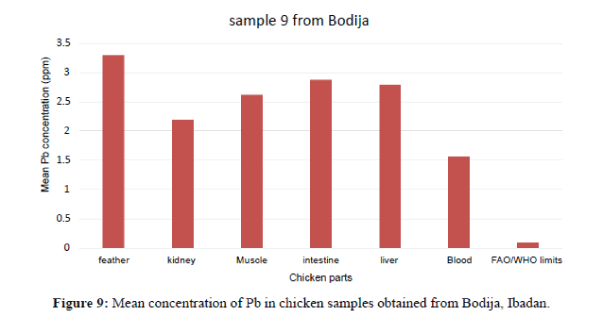
Figure 4 shows that the liver have the highest Pb concentration compared to other tissues and organs of chicken. The order of Pb increase was: feather<blood<intesti ne<kidney<blood<liver. There is significant variation among the tissues and organs of chicken exceeded the permissible limit set by FAO/WHO (2002) of (0.1 ppm). In sample 5, the kidneys have the highest Pb concentration compared to other tissues and organs. The order of Pb increase was: intestine <blood<feather<muscle<liver<kidney. The kidney showed significant variation. They exceeded the FAO/WHO limit (0.1 ppm) as shown in Figure 5. The blood had the highest Pb concentration compared to other tissues and organs. The order of Pb increase was: muscle<liver<kidney<intestine< feather<blood. There was a significant variation among the tissues and organs of sample 6 obtained from Ojoo. The Pb content of liver and muscle was within the limit (0.1 ppm) set by FAO/WHO. The Pb content of the intestine, blood, kidney and feather are above the permissible limit shown in figure 6. The blood had the highest Pb concentrations compared to other tissues and organs. The order of Pb increase was: kidne y<liver<muscle<feather<intestine<blood. The blood showed significant variation. The Pb contents in sample 7 exceeded the maximum permissible limit of FAO/WHO (2002) of (0.1 ppm) as shown in Figure 7. The feathers have the highest Pb concentration compared to other tissues and organs. The order of Pb increase was: blood<liver<kidney<intestine<mu scle<feather. They were above the permissible limit (0.1 ppm set by FAO/WHO except the blood which is within the limit as shown in Figure 8 in sample 9, all the tissue and organ Pb are above the FAO/WHO permissible limit as shown in Figure 9. The feather had the highest concentration. The order of Pb increase was: blood<kidney<muscle<liver<intest ine<feather. The results indicated that chicken meat exceeded the FAO/WHO permissible limit (0.1 ppm) and should not be consumed because of high Pb levels they contain in sample 10; all the tissue and organ Pb were above the FAO/WHO permissible limit as shown in Figure 10. The intestine had the highest concentration. The order of increase was: blood< liver<muscle<feather<kidney<intestine. There is significant variation among the tissues and organs of chicken. Therefore, should not be consumed because of high Pb levels they contain. The Pb content of the blood obtained from Sango location is much higher compared to other locations. The order of Pb increase was: Sango>Bodija>Ojoo.
Discussion
Distribution of lead (Pb) the organs and tissues of domestic chicken in Ibadan
From the results of this study, the concentrations of the Pb metal in the kidney, liver, intestine, muscle, feather and blood of the chickens was found to be statistically significant (P<0.05). The specific functions performed by these organs and tissues of the domestic chicken in the study as well as the bioaccumulative nature of the Pb metal in the system were reasons adduced for the obvious significance. Physiologically, the kidney carries out ultrafiltration and selective absorption; hence there is the tendency for it to retain the heavy metal Pb in its tissue in the absence of a chelating agent. Since the liver detoxifies and stores harmful substances, Pb cannot be an exception for it to store. The results obtained from this study indicated that Pb concentrations were variable between tissues and organs of chickens collected from different retailer markets in Ibadan City. They possess unequal distribution of Pb metal throughout their body parts whereby concentration of Pb were higher in some samples compared to others.
The concentrations of Pb in the internal organs of chickens investigated in the present study were found to be higher than the levels of Pb reported in Nigeria chicken in Awka by Okoye et al. (2015) ranges of mean concentrations of the metals in the internal organs of the chickens in mg/g were Pb; (0.046 - 0.478). The values of 0.77 ± 0.01 mg/kg reported for Pb in chicken meat consumed in Southern Nigeria (Iwegbue et al. 2012) were found to be almost similar to the levels of Pb levels obtained in the present study. However, the findings of the present study are higher than the levels reported in the internal tissues of chicken with lower concentration of lead in Turkeys (Uluozlu et al. 2009). Pb levels in all the chicken muscle in this present study exceeded the values reported in the chicken muscle in Southern Nigeria (Uche et al. 2012). A high concentration of Pb in the blood of chickens investigated in the present study corresponds with the values of 0.861 ± 0.27 ug/g reported for in chicken meat consumed in Malaysia (Salawa et al. 2012). Fluctuating levels of Pb in the blood may reflect the diet and mobilization quantities stored in the tissue or respiratory exposure to pollutants (Rattner et al. 2008). Pb concentrations in chicken feather obtained in the present study corresponds with the results reported in Malaysia (Salawa et al. 2012). The fluctuation of the Pb concentration in feathers reflect the condition and diet during the period of feather growth, i.e., when the feather is connected to blood vessel and metals are incorporated in the keratin structure (Dauwe et al. 2000). In addition, birds are able to eliminate an important part of their body burden of some heavy metals in their feather during the molting period (Burger 1993). Feathers are useful in environmental studies to evaluate the ecological health of the local ecosystem and can be used as a non-destructive biomonitoring of the tool (Burger et al. 2007). Feathers found in this present study occupy the second rank of Pb concentration, which were investigated in this study, making them suitable bioindicators for metal accumulations as well as excretory deposition. Thus, lead has no biological functions in the body instead it is a toxic element which cause severe health risks to chicken and human consumers (Commission of the European Communities 2001).
Variation of Pb concentration in the organs and tissues
The results in this study indicate that the concentration of Pb varies between localities. The concentration of lead in the kidney samples at Bodija locality is significantly higher compared to other localities. Pb levels in blood samples at Sango locality is much higher compared to other localities. At Bodija Market, concentration of Pb in feather, kidney and liver is higher followed by Ojoo locality. The variation probably result from different feeds, whereas the chickens are exposed to high automobile fuel combustion where lead accumulate. The results show the relationship between lead concentration in chicken meat which is locally contaminated from drinking water and the soil.
Conclusion
The results obtained show the presence of lead metal in chicken meat that is widely consumed in Ibadan. The findings of this study have shown that chicken has the potential to accumulate Pb in their tissues. The high concentrations of Pb in the edible parts of chickens can pose a serious health risk to humans who consume them as excess lead is known to reduce the cognitive development and intellectual performance in children and to increase blood pressure and cardiovascular disease incidence in adults. The results showed that lead concentrations are dependent on the sampling locations, the organs, the tissues and chicken samples. Since toxic Pb residues were found to be in high levels in liver and muscle of sample 1 obtained from Bodija market are above the permissible limit of 0.1 ppm set by FAO/WHO, these organs are considered unsafe for human consumption whereas the kidney and intestine are within the recommended limit hence, safe for consumption. In sample 2, 9 and 10 (Bodija), all the edible parts of chicken meat are above recommended limit, they are considered unsafe for human consumption. In sample 3 and 4 (Ojoo), the chicken meat are considered unsafe for human consumption since they are above the recommended limits (0.1 ppm) set by FAO/WHO. In sample 5, 6 and 7 (Sango), Pb residues were within the recommended limit so they are considered safe for human consumption as well as in sample 8 (Bodija). However, consistent surveillance and monitoring should be used as its bioaccumulation could lead to serious human health problems among consumers. In addition, excessive consumption of chicken meat chicken from Pb contaminated environments should be discouraged.
In addition, garlic could be recommended to antagonize Pb toxicity, as garlic contains chelating compounds capable of enhancing the elimination of Pb. Garlic feeding can be exploited to safeguard human consumers by minimizing Pb concentrations in chicken meat which had been grown in a Pb polluted environment. Medical chelation program and consumption of fruits and vegetables rich in vitamin-C could aid in the excretion of Pb from humans that have accumulated the heavy metal in their systems. Use of leaded products in Nigeria should be banned because of its toxic effect on humans and animals. Industries should be sited far from poultry farms because of the risk of contamination of food, air and water by this metal. The need to identify sources of pollution in the local places of raising poultry in Ibadan City and reduction of these sources through direct supervision as well as activating the role of censorship on imported foodstuffs and the detection of heavy metals due to their influence on human health is necessary for preventing lead exposure.
In general, the poultry feeds, water source and ambient air quality should be strictly monitored through biomonitoring programs for lead to avoid exposure to this highly toxic heavy metal.
References
Akan, J.C., Abdulrahman, F.L., Sodipo, A.L. andChiromaY.A., 2010. Distribution of Heavy Metals in the Liver, Kidney and Meat of Beef, Mutton, Caprine and Chicken from KasuwanShanu Market in Maiduguri Metropolis, Borno State, Nigeria. Research Journal of Applied Sciences, Engineering and Technology, 2: 743-748.
ATSDR., 2007. Toxicological profile for lead. Atlanta, GA: US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
Babalola, O.O., Ojo, L.O. and Aderemi, M.O., 2005. Lead levels in some biological samples of automechanics in Abeokuta, Nigeria. Indian Journal of Biochemistry and Biophysics, 42: 401-403.
Burel, S.A. and Valat, C.,2009. The Effect of the Feed on the Host Microflora Interactions in Poultry: An Overview. Proceedings in International Symposium in Sustainable Animal Production: The Challenges and Potential Developments for Professional Farming for 13th Congress of the International Society for Animal Hygiene, 72: 645-652.
Burger, J., 1993. Metals in avian feathers bioindicators of environmental pollution. Reviews in Environmental Contamination and Toxicology, 55: 203-311.
Burger, J. andGochfeld, M.,2000. Effects of lead on birds (Laridae): A review of laboratory and field studies. Journal of Toxicology and Environmental Health,3: 59.
Burger, J. andEichhorst, B., 2007. Heavy metals and selenium in Grebe feather from Agassiz national wild life refuge in Northern Minnesota. Archives of Environmental Contamination and Toxicology, 53: 442-449.
Church, M.E., Gwiazda, R., Risebrough, R.W, Sorenson, K., Chamberlain, C.P, Farry, S., Heinrich, W., Rideout, B.A. and Smith, D.R., 2006. Ammunition is the Principal Source of Lead Accumulated by California Condors Re-Introduced to the Wild. Environmental Science and Technology, 40: 6143-6150.
Cooper, G.P., Suszkiw, J.B. andManalis, R.S., 1984. Heavy metals: effects on synaptic transmission, Neurotoxicology, 5: 247-266.
Demirbas, 1999. Proximate and Heavy Metal Composition in Chicken Meat and Tissue. Food Chemistry, 67: 27-31.
Demirezen, O. andVrula, K., 2006. Comparative Study of Trace Elements in Certain Fish, Meat and Meat Products. Food Chemistry, 32: 215-222.
Dekofehiniti, O.O., Omotoyi, I.O., Oloremu, A.G. and Abayomi, T.G., (2012). Heavy Metals Distribution and Lipid Profile in the Stomach of Cow Grazed in Akugbe-Akoko, Ondo State Nigeria. African Journal of Biochemical Research, 6: 146-149.
Diamond, G.L., 2005. Risk Assessment of Nephrotoxic Metals. In: Tarloff, J. and L. Lash (Eds.), The Toxicology of the Kidney, CRC Press, London.
Doneley B., 2004. Treating liver disease in the avian patient. Seminars in avian and exotic pet medicine, 13: 8-15.
Duruibe, J.O., Ogwuegbu, M.O.C. andEgwurugwu, J.N.,2007. Heavy metal pollution and human biotoxic effects. International Journal of Physiological Sciences, 2: 112-118.
Eisler R., 1998. Lead Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review.
El-Neweshy, M. and El-Sayed, Y.,2011. Influence of vitamin C supplementation on lead-induced histopathological alterations in male rats. Experimental Toxicology and Pathology, 63: 221-227.
European Commission, 2006. Council Regulation (EC) No. 1881/2006 of 20/12/2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union: 34-38.
(2002).Codex Alimentarius, Schedule 1 of the proposed draft Codex general standards for contaminants and toxins in food. Joint FAO/WHO Food Standards Programme, Codex Committee, Rotterdam.
Flegal, A.R. and Smith, D.R., 1995. Measurements of Environmental Lead Concentration and Human Exposure, Reviews in Environmental Contamination and Toxicology, 143:1-45.
Flora, S.J.S., Flora, G. andSaxena, G., 2006. Environmental Occurrence, Health Effects and Management of Lead Poisoning. In: Casas, S.B., Sordo, J., (Eds.), Lead: Chemistry, Analytical Aspects, Environmental Impact and Health Effects, The Netherlands.
Franson, J.C.,1996. Interpretation of tissue lead residues in birds other than waterfowl. In: Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations, Beyer, W.J, Heinz, G.H., Redmond-Norwood, A.W.( Eds.).Lewis Publishers.
Garaza, A. and Soto, E., 2006. Cellular mechanisms of lead neuro toxicity. Medical Science Monitoring, 12: 57-65.
George, A.M., 1999. Lead Poisoning Prevention and Treatment: Implementing a National Program in Developing Countries (pp. 17-97; 203-309).The George Foundation, Bangalore.
Gidlow, D.A., 2004. Lead toxicity. Occupational Medicine, 54: 76-81.
Goldstein, G.W., Asbury, A.K. and Diamond, I., 1974. Pathogenesis of lead encephalopathy: uptake of lead and reaction of brain capillaries. Archives of Neurology, 31: 382-389.
Goyer, R.A. andRyne, B., 1973. Pathological effects of lead, International Reviews in Experimental Pathology, 12:1-77.
Gurer, H. andUruc, N., 2000. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic. Biol. Med.,29: 927-945.
Hassan, A.R., Saleh, M., Sobih, M., Wilson, S. and Reddy, P., 1998. Effects of Some Heavy Metals Pollutants on the Performance and Immune System of Chicks. Poultry Science, 77: 24-30
Hawkes, S.J., 1997. What is a heavy metal? J. Chem. Edu., 74: 1374-1378.
Herman, D.S., Geraldine, M. andVenkatesh T., 2007. Case report: Evaluation, diagnosis, and treatment of lead poisoning in a patient with occupational lead exposure: a case presentation. JOccup. Med. Toxicol., 2: 7-10.
Ibrahim, N.M., Eweis, E.A., El-Beltagi, H.S. andYasmin, E.,2012. Effect of lead acetate toxicity on experimental male albino rat. Asian Pac. J. Trop. Biomed., 2: 41-46.
(2001). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc, Washington DC.
(1994). Environmental Health Criteria 155: Biomarkers and Risk Assessment: Concepts and Principles, Geneva, World Health Organization.
(1995).Environmental Health Criteria 165: Inorganic Lead. Geneva,( pp. 29-220).World Health Organization.
Iwegbue, C.M.A., Nwoje, G.E. andIyoha, E.H., 2008. Heavy Metal Residues of Chicken Meat, Gizzard and Turkey Meat Consumed in Southern Nigeria. Bulgarian Journal of Veterinary Medicine, 11: 275-280.
Jarup, L., 2003. Hazards of heavy metal contamination. British Med. Bull., 68: 167-182.
Jeffery, M.L., 2003. Cadmium and lead in tissues of mallard (Anasplatyrnynchos) and wood ducks (Aix sponsa) using the Illinois River (U.S.A.). Environmental Pollution, 122: 177.
Jukna, C., Juckna, V. andSiugzdaite, J., 2006. Determination of heavy metals in viscera and muscles of cattle. Bulg J. Vet Med 9: 35-41.
Lead., 2001. Air Quality Guidelines-Second Edition. Chapter 6.7, WHO Regional Office for Europe, Copenhagen, Denmark.
Lin, S., Hwang, S., Marshall, E., Stone, R. and Chen, J., 1996.Fertility rates among lead workers and professional bus drivers: A comparative study. Ann. Epidemiol., 6: 201-208.
Maas, R., Patch, S., and Parker, A., 2002. An Assessment of Lead Exposure Potential from Residential Cutoff Valves. Journal of Environmental Health 65: 9.
Maboeta, M.S., Reinecke, A.J. andReinecke, S.A., 1999. Effects of low levels of lead on growth and reproduction in the Asian earthworm Perionyxexcavatus. Ecotoxicology and Environmental Safety 44: 236-240.
Marcus, A.H. and Schwartz, J., 1987. Dose - response curves for erythrocyte protoporphyrinVs blood lead: effects of iron status.
Mariam, I.S. andNagre, S., 2004. Distribution of Some Trace and Macro Minerals in Beef, Mutton and Poultry. International Journal of Agric and Biology, 6: 816-820.
Marino, M. and Hardission, A., 2006. Lead and Cadmium in meat and meat product consumed by the population in Tenerife Island, Spain. Food Additives and Contaminants, 23: 757-763.
Massaro, E.J., 1997. Handbook of Human Toxicology. CRC Press LLC, New York.
Minnema, D.J., Michaelson, I.A. and Cooper, G.P., 1988. Calcium efflux and neurotransmitter release from rat hippocampal synaptosomes exposed to lead, Toxicol. Appl. Pharmacol., 92: 351-357.
Miranda, M., Lopez-Alonso, M., Castillo, C., Hernadez, J. and Benedito, J.L., 2005. Effect of Moderate pollution on toxic and trace metal levels in calves from a polluted area of Northern Spain. Environ Int., 31:543-548.
Nasser, M.A., Shabir, A., Asia, B., Aisha, K.R. and Ahteram, B., 2013. Distribution of Heavy Metals in the Liver, Kidney, Heart, Pancreas and Meat of Cow, buffalo, Goat, Sheep and Chicken from Kohat market Pakistan.Life Science Journal, 201: 10.
Okoye, P.A.C., Ajiwe, V.I.E., Okeke, O.R., Ujah, I.I., Asalu, U.B. andOkeke, D.O., 2015. Estimation of Heavy Metal Levels in the Muscle, Gizzard, Liver and Kidney of Broiler, Layer and Local (Cockerel) Chickens Raised within Awka Metropolis and Its Environs, Anambra State, South Eastern Nigeria.Journal of Environmental Protection 6: 609-613.
Onianwa, P.C., Lawal, J.A., Ogunkeye, A.A. andOrejimi, B.M., 2000. Cadmium and nickel composition of Nigerian foods, J. Food. Compos. Anal. 13: 961-969.
Pain, D.J., 1996. Lead in Waterfowl. In: Environmental Contaminants in Waterfowl: Interpreting Tissue Concentrations (Beyer, W.J, Heinz, G.H., and Redmon-Norwood, A.W., eds.).Lewis Publishers: 251-264
Rattner, B.A., Franson, J.C., Sheffield, C.I., Goddard, N.J., Leonard, D.S. and Wingate, P.J., 2008.Sources and Implications of Lead Ammunition and Fishing Tackle on Natural Resources. Wildlife Society Technical Review.
Ridley, R. and Channing, J., 1999. Workplace safety, (Oxford: Butterworth Heinemann, Vol. 4 of the Safety and Work Series: 435.
Salwa, A.A., Shuhaimi-Othman, M. and Abdulsalam, B., 2012. Assessment of Trace Metals Contents in Chicken (Gallus gallusdomesticus) and Quail (Coturnixcoturnix japonica) Tissues from Selangor (Malaysia). Journal of Environmental Science and Technology, 5: 441.
Sidhu, P. and Nehru, B., 2004. Lead intoxication: Histological and oxidative damage in rat cerebrum and cerebellum. J. Trace Elem. Exp. Med., 17: 45-53.
Sileo, L., Beyer, W.N. and Mateo, R., 2003. Pancreatitis in wild zinc poisoned waterfowl. Avian pathol32: 655.
Simons, T.J.B. andPocock, G., 1987. Lead enters bovine adrenal medullary cells through calcium channels, J. Neurochem, 48: 383-389.
Stellman, J.M. andOsinsky, D., 1997. Encyclopaedia of Occupational Health and Safety (pp. 63.19-63.24).4th Edition, International Labour Organization (ILO), Geneva.
Taib, N.T., Jarrar, B.M. and Mubarak, M., 2004. Ultrastructural alterations in hepatic tissues of white rats (Rattusnorvegicus) induced by lead experimental toxicity. Saudi J. Biol. Sci., 11: 11-20.
Timbrell, J.A., 2000. Principles of Biochemical Toxicology. (3rd Edn).,Taylor and Francis Ltd., UK.
Uche, I.O., Leo, C.O. and Nick, C.O.,2012. Assessment of metal pollution in muscles and internal organs of chicken raised in Rivers State, Nigeria. Journal of Emerging Trends in Engineering and Applied Sciences.
Uluozlu, O.D., Tuzen, M., Mendil, D. andSoylak, M., 2008. Assessment of Trace Element Contents of Chicken Products from Turkey. Journal of Hazardous Materials, (163), 2-3: 982-987.
2007. US Environmental Protection Agency, Federal Water Pollution Control Act. November 27, 2002.
2006. U.S. Geological Survey, Apparent Consumption vs. Total Consumption-A Lead-Acid Battery Case Study.
1999. WHO,Hazard prevention and control in work environment: Airborne Dust, WHO, Geneva 1999/SDE/OEH/99.14.
Young, R.A., 2005. Toxicity Profiles: Toxicity Summary for Cadmium, Risk Assessment Information System. University of Tennessee, Nashville, TN, USA.