Research Paper - Asian Journal of Biomedical and Pharmaceutical Sciences (2018) Volume 8, Issue 64
Applying Experimental Design and Optimization of Studying the Hydrophilic Matrix Sustained-Release Tablets of Felodipine
Duyen Nguyen Thi Thanh1*, Duc Hoang Van2, MinhVo Xuan1, Xuan Dam Thanh1 and Tung Bui Thanh31Department of Industrial Pharmacy, Hanoi University of Pharmacy, 13-15 Le Thanh Tong Street, Hanoi, Vietnam
2Drug Administration of Vietnam, Hanoi, Vietnam
3School of Medicine and Pharmacy, Vietnam National University, Ha Noi, 144 Xuan Thuy, Cau Giay, Ha Noi, Vietnam
- *Corresponding Author:
- Duyen Nguyen Thi Thanh
Department of Industrial Pharmacy,
Hanoi University of Pharmacy, Hanoi, Vietnam
E-mail: nglamduyen@gmail.com
Accepted on March 23, 2018
Abstract
Objective: Felodipine is a calcium channel blocker used for hypertensive and unstable angina treatments. The sustained release formulations of felodipine have advantages of achieving good therapeutic effects, increasing a bioavailability, decreasing dosing times per day and reducing side effects. The aim of our study was to study the formulation screening, then use an experiment design for formulating a hydrophilic matrix sustained release Tablet of felodipine. Methods: The optimization process had the influences of the chosen excipients (including HPMC E4M, HPMC E15LV) on the drug release. Three dependent variables were percentages of released felodipine at the sampling times 2 h, 6 h, 10 h (Y2, Y6, Y10, respectively). Results: The release profile from the optimized formula almost met the predicted release profile and similar to reference Tablets. The kinetics of drug release the optimized Tablets and reference Tablets were also followed the Korsmeyer-Peppas model. Conclusion: The optimized formula was obtained, and the in vitro release profile was similar to the predicted profile and reference Tablets.
Keywords
Felodipine, Solid dispersion system, Sustained-release, HPMC, Hydrophilic matrix.
Introduction
Felodipine is a calcium channel blocker used for hypertensive and unsTable angina treatments. Felodipine is a drug belongs to BCS Class II having low aqueous solubility and high permeability. Although felodipine was almost completely absorbed through the gastrointestinal tract and a long half-life (11-25 h), felodipine has very low water solubility, and is rapidly metabolized by the liver. The results that the bioavailability of felodipine traditional dosage form is not high (15-20%). Oral drug delivery is the most preferable route of drug delivery due to advantages of administration. Nowadays, the sustained release dosage forms have made significant progress in terms of efficacy and patient compliance as compared with conventional dosage forms. Therefore, felodipine is formulated in the sustained release form to maintain sTable blood levels and to reduce side effects, increase a bioavailability, decrease dosing times per day.
In the world, the sustained release dosage forms of felodipine have been studied by many scientists [1-4]. Because of very low water solubility of felodipine, it should increase the solubility and dissolution of felodipine before preparing the extended release system [5]. Following our results of studying on increasing the solubility and dissolution of felodipine by preparing the felodipine solid dispersion system. In this work, we apply experimental design and optimization of studying the hydrophilic matrix sustained- release Tablets of felodipine of 5 mg.
Materials and Methods
Materials
Felodipine (China), HPMC E4M CR, HPMC E15LV (Dow), magnesium stearate, Aerosil, lactose monohydrate (China), all these ingredients met the requirements of pharmaceutical or P.A. grade.
Methods
Preparation of felodipine Tablets: by direct compression. Lactose monohydrate and HPMC were sieved through No. 250 screen; magnesium stearate and Aerosil were sieved through No. 180 screen. Weigh the ingredients according to the preparation formula. The felodipine solid dispersion system, lactose monohydrate, HPMC was blended to make a homogenous mixture. Then, this mixture was lubricated with magnesium stearate and Aerosil. Tablets were compressed using a Tableting machine (Korsch-Germany). The obtained Tablets had the average weight of 215 mg, diameter of 9 mm, convex cylinder shape.
Coating method: HPMC E6, HPMC E15 are polymers, PEG 4000 is plasticizer. Charge of 1.5 kg includes 1000 felodipine Tablets and placebo Tablets. Using KBC-BP-05 coating machine with the parameters are follow: inlet temperature of 50-55°C, outlet temperature of 40-45°C, spray rate: 30 mL/ min, atomizing air pressure of 1 bar, rotational speed of pan of 8 rpm.
Dissolution test: Dissolution testing was performed according to an adapted method from the USP 34th Edition [6]. Using the ERWEKA DT 6000 dissolution tester (Germany) with stationary Tablet basket. Medium: pH 6.5 phosphate buffer with 1% sodium lauryl sulfate: 500 mL; Apparatus 2:50 rpm; temperature of medium: 37 ± 0.5ºC. Place each Tablet in to the stationary basket (test 1-USP 38) which was immersed in to the medium. The samples were withdrawn at predetermined times and equivalent amounts of medium were added into the vessel to maintain the sink condition. Samples were then centrifuged at 4000 rpm for 10 min. The centrifugation method was used instead of filtration due to the adsorption of felodipine to various filter papers. Felodipine in supernatants were analyzed by UV absorbance measurement method, at 363.2 nm, blank sample is pH 6.5 phosphate buffer with 1% sodium lauryl sulfate.
Tolerances: The percentages of the labelled amount of C18H19Cl2NO4 dissolved at the times specified conform to Acceptance: 2 h: 10-30%; 6 h: 42-68%; 10 h: ≥ 75%. Plendil ® 5 mg (AstraZeneca, Sweden) was chosen as reference Tablet.
Comparison of dissolution profiles: A similarity factor can be defined as:
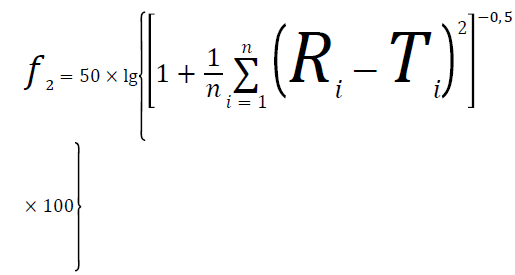
In the equation above f2 is the similarity factor, n is the number of sampling time points, Rt is dissolution at time point t of the reference, and is dissolution at time point of the test.
The evaluation of similarity is based on the conditions of: A minimum of three time points; 12 individual values for every time point; not more than one mean values of >85% dissolved and that the standard deviation of the mean should be less than 10% from the second to last time point. F2 value between 50 and 100 suggests that two dissolution profiles are similar. Assessment of drug release kinetic model: Data analysis was applied of the Wagner, zeroorder, Weibull, Higuchi, Hixson- Crowell, Korsmeyer-Peppas, Hopfengerg, Hill, Weibull, Makoid-Banakar model. Experimental design: Experimental design and optimization of formulation were performed using Model 8.0 software. Data were analyzed statistically by onewayanalysis of variance (ANOVA) using Sigma Plot 10.0 program (Systat Software Inc)and by the Student’s t-test (level of significance for p<0.05).
Results and Discussion
The dissolution profile of felodipine from Tablet reference (Plendil ® 5 mg: AstraZeneca, Sweden). The results are presented in Table 1.
| Time (h) | 2 h | 4 h | 6 h | 8 h | 10 h |
|---|---|---|---|---|---|
| Dissolved Percent of FDP (%) | 15.46 | 30.61 | 49.64 | 68.02 | 89.8 |
| RSD | 0.21 | 3.22 | 1.13 | 3.31 | 1.24 |
Table 1: The dissolved percent of felodipine from the reference Tablets (n=12, x¯).
The release kinetics of reference Tablets were analyzed by using Math CAD software 14 showed that: which were complied with Korsmeyer-Peppas kinetics model, has the smallest AIC of 2.418 and high adjusted correlation coefficient (R2 adjusted=0.999). The study of sustained release Tablet formula of felodipine was designed with hydrophilic matrix system: Through reference documents and experimental results, we selected the following excipients to study the preparation of felodipine 5 mg sustained release Tablets: HPMC E4M CR, HPMC E15 LV: controlled excipients which swell to form gel barrier in water and slow the drug release. Lactose monohydrate: filler dissolved and corroded. Aerosil and magnesium stearate: lubricants.The solid dispersion system (SDS) of felodipine includes felodipine: PEG 4000: PVP K30: Poloxamer=1:2:4:3 (FDP: PEG4000: PVP K30: PLX=1:2:4:3) was prepared by melting method. This SDS conforms to desired specification such as Appearance: White to yellowish powder; assay: 9-11%; solubility: ≥ 650 μg/ml (saturated concentration of felodipine in poloxamer 1% solution); dissolution: 1 h: ≥ 80%. The basically compositions of felodipine 5 mg sustained release Tablet are designed:
| No | Ingredients | mg/Tablet |
|---|---|---|
| 1 | Felodipine SDS | 50 mg (a quantity equivalent to 5 mg of felodipine) |
| 2 | HPMC E4M CR | change |
| 3 | HPMC E15LV | change |
| 4 | HPMC K4M CR | 10.75 |
| 5 | Magnesiumstearate | 2 |
| 6 | Aerosil | 1 |
| 7 | Lactose monohydrate | Â enough to 215 mg |
Table 2. The basically compoitions of felodipine 5 mg suatained release Tablet .The formula were designed screeing in Table 3.
When the HPMC E4M CR increased from 53.75 to 75.25 mg (mg/Tablet) or 25 to 35% (w/w), the drug release rate decreased. As HPMC E4M CR has a high viscosity, form gel layer, when the thicker gel layer was grown, the larger diffusion layer was formed, the slower erosion process was done, slowing down the drug release rate. In CT3 composition, when the polymer concentration is too low which will not be able to create a release controlled gel, the matrix was dissolved and eroded completely only after 8-9 h, while in CT1 and CT2 Tablets after 10 h, the matrixes were still remaining the drug release (amount of drug was released only about 50%).
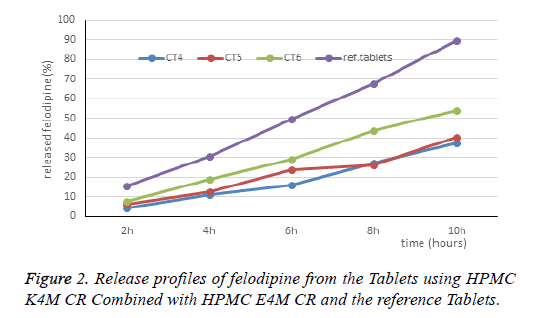
Because of that, the next step is to using more another polymer to improve the gel strength of the matrix. Influence of HPMC K4M CR on the release characteristics of felodipine when combined with HPMC E4M: The CT4, CT5 and CT6 formulas are designed in Table 2. The results of dissolution are presented in Table 3 and Figure 2.
| No | Ingredients | mg/Tablet |
|---|---|---|
| 1 | Felodipine SDS | 50 mg (a quantity equivalent to 5 mg of felodipine) |
| 2 | HPMC E4M CR | change |
| 3 | HPMC E15LV | change |
| 4 | HPMC K4M CR | 10.75 |
| 5 | Magnesiumstearate | 2 |
| 6 | Aerosil | 1 |
| 7 | Lactose monohydrate | Â enough to 215 mg |
Table 2. The basically compoitions of felodipine 5 mg suatained release Tablet .The formula were designed screeing in Table 3.
When adding HPMC K4M CR of 10.75 mg (mg/Tablet) to the Tablet, the drug release rate dropped sharply. Because HPMC K4M CR has a higher hydroxypropyl substitution ratio than the HPMC E4M CR, it is hydrated quickly and forms durable gel layer which control the drug release with the diffusion mechanism rather than the dissolution erosion mechanism.
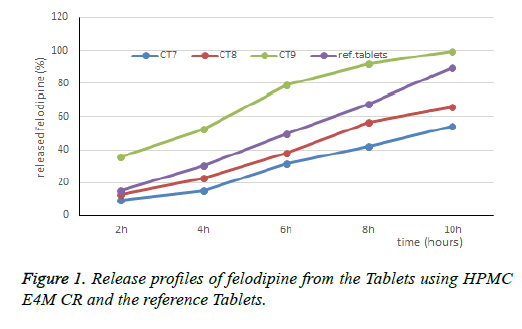
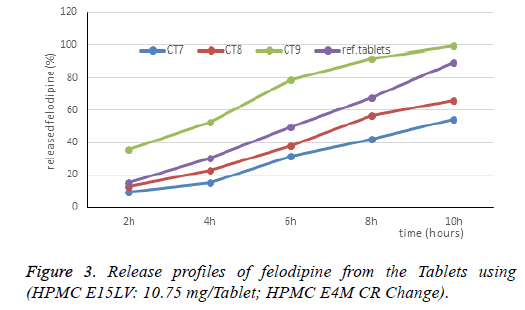
Meanwhile, the HPMC E4M controls the drug release according to the gel dissolution erosion mechanism. Therefore, when combined HPMC E4M CR with HPMC K4M CR will inhibit the drug release more suitable. The next study is the addition of a low viscosity polymer to reduce the viscosity of the gel layer. Influence of HPMC E15LV on the release characteristics of felodipine when combined with HPMC E4M: The CT7, CT8 and CT9 formulas are designed in Table 3. The results of dissolution are presented in Table 3 and Figure 3.
| Formula | HPMC E4M CR (mg) | HPMC E15LV (mg) | HPMC K4M CR (mg) | The dissolved percent of felodipine from SR Tablets(n=6,x) | ||||
|---|---|---|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 8 h | 10 h | ||||
| CT1 | 75.25 | 6.96 | 13.33 | 27.05 | 30.76 | 41.99 | ||
| CT2 | 64.5 | 9.12 | 18.45 | 32.22 | 47.73 | 57.55 | ||
| CT3 | 53.75 | 27.62 | 41.05 | 63.33 | 78.03 | 99.14 | ||
| CT4 | 75.25 | 10.75 | 4.53 | 11.01 | 16.24 | 27.28 | 37.3 | |
| CT5 | 64.5 | 10.75 | 6.32 | 12.69 | 24.03 | 26.11 | 40.69 | |
| CT6 | 53.75 | 10.75 | 7.81 | 19.04 | 29.32 | 43.67 | 54.2 | |
| CT7 | 75.25 | 10.75 | 9.44 | 15.06 | 31.68 | 41.93 | 54.69 | |
| CT8 | 64.5 | 10.75 | 12.57 | 22.75 | 37.79 | 56.74 | 65.86 | |
| CT9 | 53.75 | 10.75 | 35.89 | 52.59 | 78.76 | 92.06 | 99.23 | |
| CT10 | 64.5 | 6.45 | 10.2 | 18.53 | 33.54 | 48.62 | 60.14 | |
| CT11 | 64.5 | 17.2 | 14.19 | 23.22 | 35.37 | 60.19 | 75.45 | |
| CT12 | 62.78 | 18.92 | 15.87 | 24.11 | 37.04 | 67.3 | 86.28 | |
| CT13 | 60.39 | 21.31 | 15.56 | 22.03 | 36.92 | 64.53 | 82.7 | |
| CT14 | 57.47 | 24.23 | 14.21 | 27.25 | 39.01 | 63.38 | 85.18 | |
| CT15 | 59.48 | 17.92 | 15.24 | 28.94 | 41.69 | 70.82 | 90.78 | |
| CT16 | 56.17 | 16.93 | 17.43 | 26.94 | 54.88 | 86.26 | 101.83 | |
| CT17 | 57.2 | 20.2 | 17.51 | 30.31 | 46.5 | 69.45 | 86.62 |
Table 3. Design of FDP SR Tablet formula and the results of dissolution influence of HPMC E4M CR on the release characteristics of felodipine. The CT1, CT2 and CT3 formula re designed in Table 3. The results of dissolution are presented in Table 3 and Figure 1.
When adding HPMC E15LV 10.75 mg to the Tablet, the drug release speed has improved. When combined HPMC E4M CR (2% polymers in water solution have a viscosity of about 40000 mPa.s with HPMC E15LV (2% polymers in water solution have a viscosity of about 15 mPa.s), the polymer mixture has a lower viscosity compared to HPMC E4M CR, low viscosity helps to loosen the gel, which makes the gel more susceptible to corrosion and facilitates the drug diffusion through the gel layer.
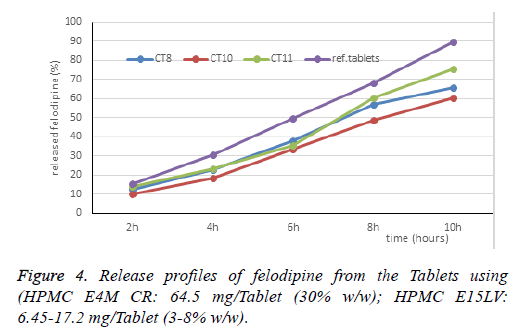
Thus, the research combining HPMC E15LV and HPMC E4M gives suitable results, selecting CT8 for further study. The HPMC E4M ratio is 30%, the change in the HPMC E15LV ratio is 3%, 5%, 8%. The dissolution profile of the CT10, CT11 Tablets are shown in Table 3 and Figure 4.
Drug release rates increased when increased amount of HPMC E15LV (from 3% to 8%). Increasing the ratio of HPMC E15LV increases the matrix ratio in the Tablet (from 33% to 38%), and decreases the viscosity of the gel. When increased matrix ratio (about 5%) increases the gel thickness that reduces the drug release, but the sharp decrease in viscosity causes the gel more loosely. In that case, increasing the drug release was more than playing role in reducing the drug release. As a result, the higher amount of HPMC E15LV was, the higher drug release was. The results showed that the dissolution profile of CT11 was close to the reference Tablets, but at 6 h, amount of released drug was still failed (35.37% compared with 42-68%). CT11 was selected for further study.
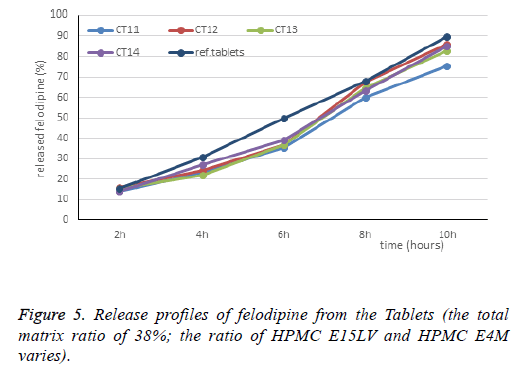
The CT12-CT14 formulas is based on the principle of keeping the total matrix ratio of 38% (81.7 mg), increasing the HPMC E15LV, reducing the HPMC E4M to reduce the viscosity of gel. The results of dissolution are shown in Table 3 and Figure 5.
Results showed that the dissolution profiles of CT12, CT14 were close to the reference Tablets. But at 6 h, amount of released drug was still failed (37.04% and 39.01% respectively).
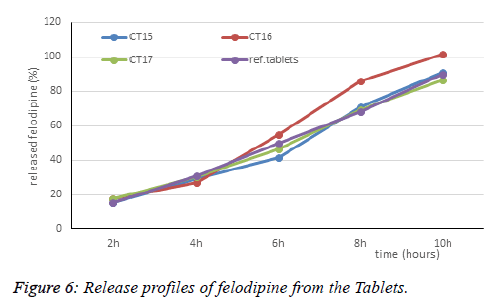
CT15, CT16 formulas is based on the principle of reducing the total matrix of 36% (77.4 mg) and 34% (73.1 mg) respectively, and the ratio of HPMC E15 and HPMC E4M is similar to CT12. CT15 Tablets had amount of released drug at 6h of 41.69%. Because of that result, CT17 was designed with the total matrix of 36%, and increasing amount of HPMC E15LV of 20.2 mg (9.4%), decreasing amount of HPMC E4M of 57.2 mg (26.6%). The dissolution data meet the requirement for drug release rate according to the US pharmacopoeia at 2 hours is 17.51% (10-30%), 6h is 46.5% (42-65%), 10h is 86.62% (>75%). Thus, based on the results of the screening study, CT17 formula was selected as the basic formula in the experimental planning application.
Notes: other excipients such as lubricants (magnesiumstearate 2 mg, Aerosil 1 mg), SDS of felodipine (the amount contains 5 mg of felodipine) are constants, lactose monohydrate: filler enough weight of Tablet (215 mg)). Dependent variables: the aim of preparation the felodipine sustained release Tablets is that the dug release profile is similar to that of reference Tablets, symbols and levels of dependent variables are showed in Table 5.
| Independent variables | Symbols | Low value (-1) | Central value (0) | High value (+1) |
|---|---|---|---|---|
| HPMC E4M (mg/Tablet) | X1 | 53.2 | 57.2 | 61.2 |
| HPMC E15LV (mg/Tablet) | X2 | 15.2 | 20.2 | 25.2 |
Table 4. Symbols and levels of independent variables.
| Variables: the dissolved percent of felodipine | Symbols | Targets | Requirements |
|---|---|---|---|
| At sampling times 2 h | Y2 | 0.1546 | 10%-30% |
| At sampling times 6 h | Y6 | 0.4964 | 42%-68% |
| At sampling times 10 h | Y10 | 0.898 | = 75% |
Table 5. Symbols and levels of independent variables.
Experimental design: this experimental design required 11 experiments in total (Table 6).
| Formula | Independent variables | The dissolved percent of FDP from SR Tablets | |||
|---|---|---|---|---|---|
| HPMC E4M mg/Tablet (X1) | HPMC E15LV mg/Tablet (X2) | 2 h (Y2) | 6 h (Y6) | 10 h (Y10) | |
| N1 | 53.2 | 15.2 | 22.1 | 61.11 | 102.5 |
| N2 | 61.2 | 15.2 | 12.63 | 46.04 | 81.57 |
| N3 | 53.2 | 25.2 | 17.2 | 53.69 | 100.07 |
| N4 | 61.2 | 25.2 | 7.53 | 29.98 | 67.16 |
| N5 | 51.544 | 20.2 | 19.84 | 61.85 | 102.33 |
| N6 | 62.856 | 20.2 | 10.67 | 41.62 | 75.01 |
| N7 | 57.2 | 13.13 | 16.8 | 51.09 | 97.59 |
| N8 | 57.2 | 27.27 | 11.24 | 44.02 | 79.49 |
| N9 | 57.2 | 20.2 | 13.23 | 49.39 | 88.02 |
| N10 | 57.2 | 20.2 | 12.57 | 46.12 | 85.12 |
| N11 | 57.2 | 20.2 | 14.55 | 49.55 | 88.86 |
Table 6. Experimental matrix and the results of dissolution.
Results and statistical analysis: the SR Tablets were prepared. After that, in vitro drug release from Tablets was tested. The results of dissolution data are showed in Table 6.
*Data analysis: The model was fitted to the data for all responses simultaneously using Model for Windows computer program.
Yi=A0+A1*X1+A2*X2+A11*X12+A22* X22+A12*X1*X2
I ∈ {2, 6, 10,}; A0, A1, A2, A11, A22, A12 are regression coefficients.
The resultant equations are given below:
Y2=13.45-4.01*X1-2.23*X2 with R2=0.96; repeatability coefficient=0.94
Y6=48.3537- 8.42443*X1- 4.18525*X2 with R2=0.93; repeatability coefficient=0.95
Y10=87.3335- 11.5606*X-5.30497*X2 with R2=0.97; repeatability coefficient=0.97
R2 and repeatability coefficient were high and approximately 1, so experimental results have statistical significance and good repeatability, the regression equations were suitable.
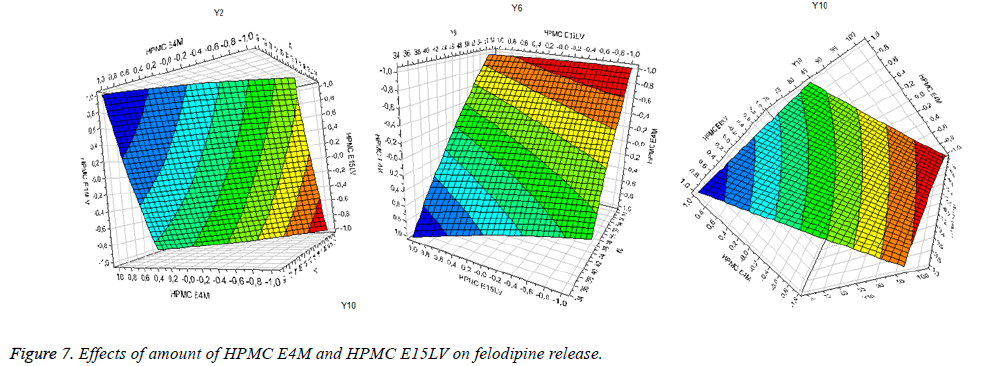
The regression equations showed that the dependent variables (Yi) depend on the independent variables (Xi) by the first order function. All coefficients of Xi are negative (-) which means that if increasing amount of HPMC E4M and HPMC E15LV decreases the dissolution rate of felodipine from Tablets.
The data analysis from the software shows that, when increased amount of HPMC E15LV from 13.13 mg to 27.27 mg with HPMC E4M retained, the percentage of drug release from the Tablet decreased: Y2 decreased from 16.8% to 11.24% (5.56%); Y6 decreased from 51.09% to 44.02% (7.07%); Y10 decreased from 97.59% to 79.49% (18.1%). When increased amount of HPMC E4M from 51.544mg to 62.856mg with HPMC E15LV retained, the percentage of drug release dropped from the Tablet: Y2 decreased from 19.84% to 10.67% (9.17%); Y6 decreased from 61.85% to 41.62% (20.23%); Y10 decreased from 102.33% to 75.01% (27.32%).
Both of these excipients are involved in drug release control. In it, the HPMC E15LV produces fast gel, which controls the release of FDP in the early hours, but this gel barrier loosely increases the corrosion rate of the matrix core - which helps regulate the speed of erosion of the gel barrier, which is special suitable for controlling the release of less soluble or poorly soluble compounds in water. HPMC E4M is a fast-gel-forming excipient in dissolution medium, inhibiting release of the drug from the matrix. Furthermore, the gel barrier produced by HPMC E4M is relatively suitable and lasts a long time in the dissolution medium to control drug release in the following hours and inhibit the drug release higher than HPMC E15LV.
The effect of the two excipients on the felodipine release from Tablets was shown more clearly in the Figure 7.
*The optimum formulation: Based on the analysis results of the Model 8.0 software, with the requirement of the output variables which have the release profiles similar to that of reference Tablets, the optimal formula was obtained with the follow input variables:
Amount of HPMC E4M=58.26 mg; amount of HPMC E15LV=16.95mg.The optimized formula from software was predicted as the following:
SDS of felodipine: 50 mg
HPMC E4M: 58.26 mg
HPMC E15LV: 16.95 mg
Magnesium stearate: 2 mg
Aerosil: 1mg
Lactose monohydrate: fill enough to 215 mg
Prepare the felodipine SR Tablets according to the optimal formulation on a scale of 1000 Tablets x 3 batches. The results of the drug release were showed in Table 7.
| Samples | 2 h | 4 h | 6 h | 8 h | 10 h | f2 ref | f2 pre |
|---|---|---|---|---|---|---|---|
| Reference | 15,46 | 30,61 | 49,64 | 68,02 | 89,80 | 100 | |
| Predicted results | 14,22 | 50,84 | 87,31 | ||||
| Batch 1 | 14.52 ± 0.14 | 30.82 ± 0.39 | 48.7 ± 1.74 | 68.38 ± 2.08 | 87.95 ± 2.14 | 92.09 | 89.24 |
| Batch 2 | 14.89 ± 0.22 | 31.25 ± 0.48 | 51.09 ± 1.51 | 64.41 ± 2.34 | 86.14 ± 2.78 | 79.1 | 94.72 |
| Batch 3 | 15.15 ± 0.16 | 32.74 ± 0.33 | 50.03 ± 1.83 | 70.89 ± 2.27 | 93.33 ± 2.03 | 80.37 | 71.67 |
| Requirements | 11232 | 42-68 | = 75 |
Table 7. The felodipine release profiles from optimal Tablets and the reference Tablets (n=12, x¯ ± SD).
Notes: Predicted results: the release data from the optimal Tablet which were predicted by the software Model 8.0; f2 ref: a similarity factor when compare between dissolution profile of optimal Tablets and that of reference Tablets; f2pre: a similarity factor when compare between dissolution profile of optimal Tablets and predicted dissolution profile of optimal Tablets.
From the data in Table 7, it shows that Y2, Y6, Y10 almost conform specifications. With a value of f2 more than 70, the optimal Tablets are similar to the predicted results and f2ref more than 75 showed that the optimal Tablets have the drug release profiles similar to that of reference Tablets (Plendil ® 5 mg, AstraZeneca, Sweden). Thus, the predicted results are suitable with the experiment and the optimal Tablets meet the requirements for in vitro drug release.
Assessment the effect of the protect film on the drug release. In order to protect Tablet cores from being humidified in the air the SR Tablet cores were coated the protective film. Charge of 1.5 kg includes 1000 felodipine Tablets and placebo Tablets. The coating formula as following: HPMC E6: HPMC E15: PEG 4000: titan dioxide: talc=50:50: 15: 12.5: 37.5 with solvent mixture of ethanol and water. After coating, the average weight gain is 4.52%. The dissolution profiles from the coated Tablets and Tablet cores are shown in Table 8:
| Samples | The dissolved percent of FDP (n=6, x¯) | Hardness (N) | ||||
|---|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 8 h | 10 h | ||
| Reference Tablets | 15.46 | 30.61 | 49.64 | 68.02 | 89.8 | 76.2 ± 5.21 |
| Tablet cores | 14.52 | 30.82 | 48.7 | 68.38 | 87.95 | 45.4 ± 4.56 |
| Coated Tablets | 14.04 | 29.76 | 48.95 | 69.45 | 86.62 | 66.8 ± 8.75 |
Table 8: The hardness and the dissolution profiles.
After coating the film, the results show that the hardness of the Tablet is significantly increased, but the dissolution data over time of the Tablet cores and the coated Tablets is not different and almost the same as the reference Tablets. This shows that the effect of film to the drug release is negligible. Therefore, the film formula was selected and the coating procedure was chosen.
Assessment the release kinetic models of felodipine optimal Tablets:
Using Math CAD 14 software, the results are presented in Table 9:
| Samples | Models | The kinetic equation | R2 adjust | AIC |
|---|---|---|---|---|
| Reference Tablets | Zero order | C(t)=-5.15+9.36.t | 0.995 | 8.554 |
| Korsmeyer-Peppas | C(t)=6.374.t1.145 | 0.999 | 2.418 | |
| OptimalTablets | Zero order | C(t)=-5.69+9.24.t | 0.998 | 4.893 |
| Korsmeyer-Peppas | C(t)=6.319.t1.142 | 0.998 | 3.364 |
Table 9: The drug release kinetic models of optimal Tablets and reference Tablets.
From the results in Table 9, the reference Tablets and the optimal Tablets have the release model similar to the zero orderkinetic models and the Korsmeyer-Peppas model (all of R2 adjust are close to 1). However, the AIC of both is smallest in the Korsmeyer-Peppas model. With the Korsmeyer-Peppas model, the exponential coefficient is approximately 1, the release kinetics of the Tablets are similar to that of the zero order kinetic model. Thus, the reference Tablets and the optimal Tablets follow the Korsmeyer- peppas kinetic model because of it have the smallest AIC. The kinetic equation: reference Tablets: C(t)=6,374.t1.145; optimal Tablets: C(t)=6,319.t1.142. This model is suiTable for the sustained release dosage forms. Drug was gradually release from dosage forms and to ensure treatment concentration during the long time. This model reflects suitability the properties of the swelling, erosion matrix containing poorly-solubility substance.
Conclusions
Research on screening of formulation of sustained release felodipine Tablet. The basic formula was selected to apply the experimental planning to find the optimal formula. Based on experimental results and analysis of Model 8.0 software; it assessed the influence of input/independent variables including HPMC E4M and HPMC E15LV on the output/dependent variables (percent of drug release at the times). The optimal formulation for the FDP hydrophilic matrix Tabletswere selected and achieved the requirements of in vitro release. The release kinetic model of obtained Tablets was complied with Korsmeyer-Peppas kinetics model and similar to that of reference Tablets.
Acknowledgement
The study is completed by funding of subject Program applied research and advanced technology development to serve and protect the public health care (KC.10/11-15), subject code: KC. 10.15/11-15.
References
- Kumar, SK, Ramarao T, Bikshapathi DBRN, Jayaveera KN. Controlled release formulation development and evaluation of felodipine matrix Tablets by using hydrophobic polymers. Int J Pharm Sci Res. 2013;4:506.
- Kyeo-Re L, Eun-Jung K, Sang-Wan S, Hoo-Kyun C. Effect of poloxamer on the dissolution of felodipine and preparation of controlled release matrix Tablets containing felodipine. Archiv pharmacal res. 2008;31:1023-1028.
- Iovanov RI, Tomuta I, Leucuta SE. Influence of some formulation factors on the release of felodipine from extended release hydrophilic matrix Tablets. 2009;57:590-597.
- Kye KL, Dong SL, Yun SR, Jun HJ, Sun OJ, Sang JK. Controlled release composition comprising felodipine and method of preparation thereof. 2003.
- Karavas E, Ktistis G, Xenakis A, Georgarakis E. Effect of hydrogen bonding interactions on the release mechanism of felodipine from nanodispersions with polyvinylpyrrolidone. Europ J pharma biopharma. 2006;63:103-114.
- United States Pharmacopeial Convention, Felodipine Extended-Realease Tablets. USP 34-NF 2011;2113.