Research Article - Journal of Environmental Waste Management and Recycling (2018) Volume 1, Issue 2
An assessment on the sustainable production of construction clay bricks with spent shea waste as renewable ecological material
AN Adazabra, G Viruthagiri*, N Shanmugam
Department of Physics, Faculty of Science, Annamalai University, Chidambaram, Tamil nadu, India
- *Corresponding Author:
- G. Viruthagiri
Department of Physics, Faculty of Science Annamalai University
Chidambaram, Tamilnadu, India
Tel: +91 8778277115
E-mail: steve.jere@gmail.com
Accepted date: September 14, 2018
Citation: Adazabra AN, Viruthagiri G, Shanmugam N. An assessment on the sustainable production of construction clay bricks with spent shea waste as renewable ecological material. J Environ Waste Management and Recycling. 2018;1(2):36-46.
Abstract
The primary objective of this research is to ascertain the potential utilization of spent shea waste as renewable ecological material in sustainable clay brick manufacturing. In this regard, the particle sizes, mineralogical, thermal properties of the brick raw materials were extensively characterized from physical, spectroscopic and thermal analytical methods, respectively. Proportionate amounts of clay materials were replacement with 0%, 5%, 10%, 15% and 20 wt.% of spent shea waste to prepare modeled brick samples via manual pressing, which were fired at 900-1200 °C temperatures. Technological properties including green density, linear fired shrinkage, fired density, water absorption, apparent porosity and compressive strengths of the modeled bricks were investigated. The nature of pores and thermal conductivity of the modelled bricks were also ascertained. The various test results indicate that use of shea waste in construction could contribute to economic firing, improve the fluxing properties of raw materials, and beneficially lighten brick weights as well as enhancing their thermal insulation properties.
Keywords
Spent shea waste, ecological construction materials, fired clay bricks, technological properties, sustainability
Introduction
In recent times, the rapid pace of brick construction due to technological advancement [1,2], dwindling reserves of non-renewable natural resources [3], legislative enactments of sovereign states [4,5], declining development in energy/ electricity [6], and climate change concerns [1], have cumulatively obsoleted convectional overreliance on clay resources and provided great impetus for use of various abundant materials as additives for construction. Other fundamental concerns creating this radical paradigm shift could conceivably be linked to population increase [7], increasing relevance of sustainability [8,9], economic production procedures [10,11], energy-savings [12], environmental conservation [13] and environmental protection issues [14,15]. Thus, the increasing popularity of replacing clay resources with often abundant and low cost materials for tailoring the properties of construction bricks into desired forms is a need-of-the-hour in the brick construction industry.
In particular, use of renewable ecological materials as essential additives to construction materials, is gaining momentum among researchers around the globe nowadays. Raut et al from India presented a review on sustainable construction materials using industrial and agricultural solid waste towards highlighting the need to harness the often-unmanaged organic residue in the design of green buildings [16]. Mucahit and Sedat from Turkey replaced up to 30 wt.% of clay materials with recycled paper processing residue, a mainly organic materials, in developing lightweight bricks with beneficial reduced thermal conductivity properties at acceptable compressive strengths [17]. Muñoz et al from Chile and Spain used waste pomace generated from the winery industry to develop fired clay bricks with improved thermal insulation characteristics [18]. Bories from France, and Muñoz et al from Chile and Spain provided a comprehensive list of organic residue used as renewable ecological construction materials globally [19,20]. Thus, the issues of reusing renewable ecological materials are an evolving field of current interest.
One such renewable ecological material that is hugely generated from the shea butter/oil industry is Spent Shea Waste (SSW). The shea (Vitellaria paradoxa, C.F. Gaertn) butter industry is increasingly catching major international attention because of its significant multifunctional butter use in the pharmaceuticals, detergent and cosmetics industries [21-23]. Shea butter is also often use as cooking oil and an essential butter formulation in traditional medicines [24]. Moreover, recent revision of European Commission regulations consenting to use of substitutes for cocoa butter coupled with a number confectionery recoveries in the former soviet states have mutually occasioned an up thrust in demand for the shea butter product [25].
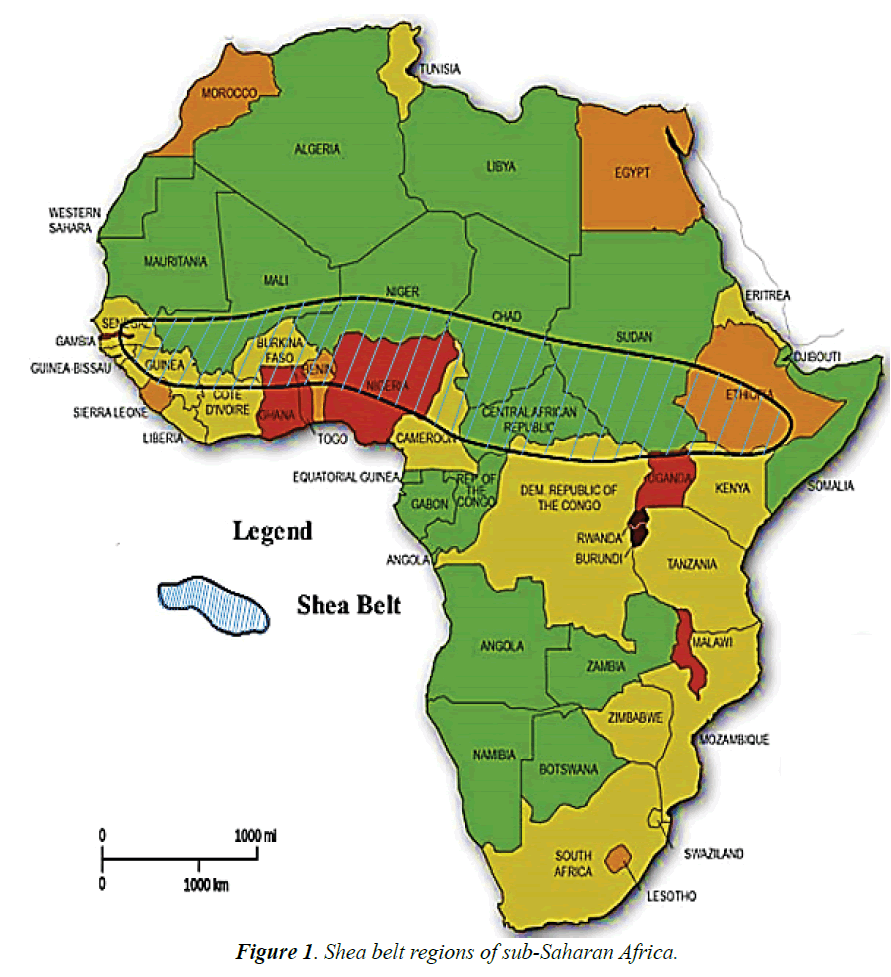
These developments have compelled most governments in the shea belt parklands (Figure 1) to commercialize the shea butter agro-industry mainly as a viable means of reducing poverty, ensuring food security, creating job opportunities and most of all, improving their non-traditional export base. Thus, the frequent generation of unprecedented levels of corollary SSW products is assuming alarming proportions. It is evident that SSW is being exported to United Kingdom for co-combustion in coal power plants conceivably because of its excellent biofuel value and relative low carbon content [26,27]. Nevertheless, the quantities involve are far less that generation capacities. Innovative ways of utilizing SSW profitably are currently limited to field trials in Africa [28].
Taking into account the substantial generation of SSW, its renewable source, the natural widespread availability of the shea tree across the African sub-Saharan regions, its comparative low cost and the beneficial influence of ecological materials on brick technological properties, SSW could be excellent target materials of choice for reuse in the construction industry advantageously. However, until now limited information exist in published literature on the reuse of SSW in the clay industries although its good calorific value is well known [25-27,29]. At present, the increasing volumes of SSW are indiscriminately damp on idle lands as a major disposal method with dire consequence to the environment.
Considering the good biofuel value of SSW, its current indiscriminate damping constitutes a major loss of valuable energy; besides, this disposal method is at variance with best environmental management practices. Therefore, the present research primarily seeks to leverage on the huge abundance of SSW for reuse as ecological construction materials towards improving the technological properties of fired clay bricks sustainably. Hence, the focus of this research is to provide baseline data on reuse of SSW as renewable ecological materials to improve the technological properties of clay bricks into various superior performing products. Therefore, it is hope that this research would create a new path of possibility on the viable synergistic reuse of SSW as indispensable construction materials towards adding value to the shea butter industry.
Highlights
• Prominent factors compelling use of ecological materials for construction stated
• A new ecological material examined for its sustainable utilization potential
• Inert, low specific gravity and biofuel of shea waste suitable for construction
Materials and Methods
Collection and pretreatment of brick raw materials
Intercontinental brick making materials of industrial high-grade standard specifications used in this research include; white clay, yellow clay and SSW. The clay resources were acquired in their pretreated powdered form from a government industrial brick making facility located at Virudachalam in the Cuddalore district of Tamilnadu state, India. The clays were pretreated via drying in a rotating drier set at 100°C for 6 hours, mechanical crushing in an industrial pulverizer into powder and then sieving through a 100-μm mesh size sieve to obtain fine homogenous powder materials that met industrial brick making high quality standard specifications.
SSW material, collected for this study, arise from the residue of shea nuts after processing for the industrial valuable shea butter. The SSW material were sampled as dry lumpy shea fragments at a major damp site located at Wakii in the Tongo district of upper east region, Ghana. These shea fragments were reduced to suitable packaging size through mechanical pounding in a domestic wooden mortar and pestle. The obtained powder was then packaged into pre-clean polyethylene bags and airlifted to spectroscopic laboratory of physics department, Annamalai University (Chidambaram, Cuddalore district, Tamilnadu state, India). Further pretreatment including mechanical ball milling, manual grounding and sieving (100-μm mesh size sieve) was carried out at the laboratory to obtain a homogeneous fine SSW powder.
Thus, the particle sizes (i.e. ≤ 100 μm) of the brick making raw materials were physically characterized. In addition, the mineralogical constituents, chemical composition and thermal dynamics of the raw materials were characterized from X-Ray Diffraction (XRD), X-Ray Fluorescence (XRF) And Thermogravimetric Differential Thermal Analysis (TG-DTA) studies, respectively. Further, the organic content and calorific value of SSW were ascertained from ultimate analysis following ASTM D-2974 standard procedure [30].
Preparation of modelled brick specimens
In accordance with the main aim of this research, the brick making raw materials were accurately weighed into five clean plastic containers following the weighed design presented in Table 1. The weighed content of each plastic container were thoroughly homogenized via mechanical attritional milling. About 13.20 wt.% of plasticity water was then added to the homogenized mixture to attain adequate granulates after applying an empirical soaking time of 1 hour. The modeled test brick specimens of wet dimensions 90 mm × 35 mm × 30 mm were shaped through uniaxial pressing at 25 Mpa of the formed granulates using laboratory type hydraulic press.
| Brick Specimens | Weighted design of mixtures | Replicates | Firing design | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute weight of mixture (g) | Clay (wt.%) | SSW (wt.%) | Rectangular brick bars | Spherical plate bricks | Batch 1 (900 °C) |
Batch 2 (1000 °C) |
Batch 3 (1100 °C) |
Batch 4 (1200 °C) |
|
| CM-SW0 | 3600 | 100 | 0 | 16 | 12 | CM-SW0 | CM-SW0 | CM-SW0 | CM-SW0 |
| CM-SW5 | 3600 | 95 | 5 | 16 | 12 | CM-SW5 | CM-SW5 | CM-SW5 | CM-SW5 |
| CM-SW10 | 3600 | 90 | 10 | 16 | 12 | CM-SW10 | CM-SW10 | CM-SW10 | CM-SW10 |
| CM-SW15 | 3600 | 85 | 15 | 16 | 12 | CM-SW15 | CM-SW15 | CM-SW15 | CM-SW15 |
| CM-SW20 | 3600 | 80 | 20 | 16 | 12 | CM-SW20 | CM-SW20 | CM-SW20 | CM-SW20 |
Table 1. Weighed and Firing designs for preparing brick specimens.
Towards presenting statistical valid test results on the technological properties of formed bricks, 16 replicates of these rectangular modeled brick specimens were prepared from each brick mixture. In addition, aliquots of the formed granulates were similarly used to prepared 12 cylindrical brick specimens of 10 mm wet thickness and 70 mm diameter, for thermal conductivity test. All prepared brick specimens were subject to open sun drying for a week. For easy identification and comparison, all the dried brick specimens were categorized into groups according to their raw materials weighed proportions and designated as CM-SW0: reference (75 wt.% white clay +25 wt.% yellow clay) standard and CM-SW5: 70 wt.% white clay +25 wt.% yellow clay + 5 wt.% SSW. The rest are CM-SW10: 65 wt.% white clay +25 wt.% yellow clay + 10 wt.% SSW; CMSW 15: 60 wt.% white clay +25 wt.% yellow clay + 15 wt.% SSW and CM-SW20: 55 wt.% white clay +25 wt.% yellow clay + 20 wt.% SSW. Where CM imply clay mixture and SW denotes shea waste.
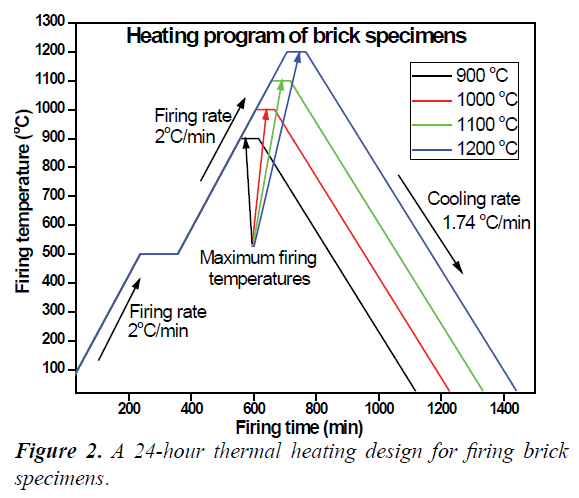
The replicates of each of these five compositional unique brick specimens were sub-grouped to into series (i.e. each series containing four replicated rectangular and three cylindrical brick specimens for all mixtures) namely Series 1, 2, 3 and 4 for sintering according to the firing design presented in Table 1. Thus, the dried brick specimens were placed in an electrical kiln for firing. A 24 hour heating cycle was adopted to sinter the brick specimens as presented in Figure 2. According to this cycle, each sub-grouped brick series were consecutively heated at a firing rate of 2°C/min up to 500°C and maintained at 500°C for 2 hours. Further heating at same 2°C/min firing rate was applied until the desired firing temperature (900-1200°C) was reached. A minimum soaking time of 1 hour was applied at each reached maximum temperature before turning the kiln off to cool to ambient temperatures by natural convection.
Technological properties test characterization of fired rectangular brick specimens
A number of tests were performed to determine the technological properties such as green density, fired shrinkage, fired density, water absorption, compressive strengths and porosity of the fired brick specimens. Prior to firing and after firing, the dimensions of each brick specimen was accurately measured using veneer calipers of least calibration 0.01 mm and its weights precisely recorded using a sensitive electronic balance of least readings 0.01 g following the EN 772-16 standard procedure [31]. Based on the data obtained from the above measurements, the Green Densities (G.D) of the dried brick specimens, Linear Shrinkages (L.S%) and Fired Densities (F.D) of the fired brick specimens were computed in line with procedures specified in the Chinese standard GB/T2542-2003 [32].
In accordance with the methods described in ASTM standard C 373-88 (ASTM C 373-88, 1988), the Water Absorptions (W.A%) and Apparent Porosity (A.P%) of the fired brick specimens were ascertained using the boiling water method. The fired brick specimens were submerged in water for 24 hours to determine their wet weights and then transferred into hot water container for a 6 hour boiling. The suspended and saturated weights of the bricks were then determined and used to calculate the water absorptions and apparent porosities of the brick specimens after four repeated measurements.
The Unitek compressor model 94100 set at a loading speed of about 1 kN/s was used to evaluate the developed compressive strengths of the brick specimens following UNE EN 772-1 standard procedure [33]. Quadruplicate measurements were done to determine the average compressive strength value of a unique brick specimen. The pore structures of the fired brick specimens containing SSW materials were examined as a function of increasing firing temperature using a using a JEOL JSM 6610 LV scanning electron microscope instrument operated in the secondary electron mode. Freshly fractured surfaces of CM-SW15 brick specimens of suitable dimensions (i.e about 4 mm × 4 mm × 1 mm) were studied as representative of all the CM-SW5-20 brick specimens. The selected specimens were stubbed to specimen holders and coated with a thin platinum layer for examination at 10 μm resolution.
Thermal conductivity of the fired cylindrical brick specimens
Thermal conductivity test on three sub-grouped fired cylindrical brick specimens were performed to ascertain the average insulation capacities of the brick specimens. The steady-state heat flow Lee’s apparatus was used to ascertain the thermal conductivity of the brick specimens at room temperatures (25°C). The diameter of the Lee’s disc used in the test was 110 mm. Therefore, the fired cylindrical brick specimens were precisely machined to fit the specifications of the disc. Three repeated measurements were then averaged as the thermal conductivity of a particular brick specimen.
Results and Discussion
Raw materials characterization
The chemical composition of brick raw materials partly determine their displayed technological properties [34,35]. The elemental compositions as well as concentration levels of the pristine raw materials (i.e. white clay, yellow clay and SSW), expressed in their common oxides form, are presented in Table 2. The concentration level of oxides present in the white clay are in the decreasing order of SiO2 > Al2O3 > CaO > Fe2O3 > MgO > ZnO as observed in Table 2. Yellow clay similarly showed SiO2 > Al2O3 > Fe2O3 > K2O > TiO2 > MgO > CaO > MnO > (Cr2O3 and V2O5) > (NiO and ZnO) as their decreasing order of oxides concentration levels. As evident in both clay materials, the high concentrations of SiO2 and Al2O3 is in agreement with their argillaceous nature [36] and the particular dominance of SiO2 in both clay materials is in consonance with most structural construction clays reported in literature [37-39].
| Oxides | Brick Making Clay | SSW |
|---|---|---|
| Al2O3 | 27.10 | 0.37 |
| BaO | 0.10 | 51.87 (µg/g) |
| CaO | 5.32 | 0.36 |
| CdO | - | 1.35 (µg/g) |
| Cr2O3 | - | 7.06 (µg/g) |
| Fe2O3 | 0.38 | 0.07 |
| K2O | - | 2.11 |
| MgO | 0.09 | 0.10 |
| MnO | 0.02 | 30.81 (µg/g) |
| NiO | - | 9.60 (µg/g) |
| PbO | - | 38.50 (µg/g) |
| SiO2 | 52.97 | 0.35 |
| TiO2 | - | 0.01 |
| V2O5 | - | 18.72 (µg/g) |
| ZnO | 0.01 | 174.19 (µg/g) |
Table 2. Chemical analysis of brick making clay and spent shea waste presented in in wt.% unless stated otherwise.
SiO2 is linked to increase in porosity and hence decrease in thermal conductivity of bricks [40]. Al2O3 is a quality-index parameter of clay materials and it reacts with SiO2 at higher firing temperatures imparting mechanical strength on the brick units via their transformation into unreactive spinels and mullites [41,42]. CaO could beneficially decrease thermal conductivity due to their reactions at calcite grain boundaries [43,44] as well as increase mechanical strength via its reactions with SiO2 to form anorthite. Nevertheless, free CaO causes significant deterioration due to its reactive nature. Therefore, recommended amount of CaO content in structural construction clay is limited to 10 wt.% [45].
Fe2O3 and TiO2 are responsible for imparting appealing color to fired bricks [46,47]. The quality of a fired brick is perceived to depend on its displayed color and hence a major determining factor of its market value [48,49]. However, high concentration levels of Fe2O3 promote efflorescence and “black-core” or “black nucleus” formation. Therefore recommended amounts of Fe2O3 in quality clay material is 10 wt.% [45]. K2O, NiO, MnO, MgO, ZnO, Cr2O3, and V2O5, are collectivity termed inorganic fluxing agents due to their economic role in lowering the liquid phase formation sintering temperature [50]. Based on the previous findings enumerated above, the high concentrations of Al2O3 and SiO2, low quantities of Fe2O3 and CaO, and good diversity of inorganic oxides (i.e. K2O, NiO, MnO, MgO, ZnO, Cr2O3, TiO2 and V2O5, suggest the potential suitability of the clay materials for use in structural construction works.
In addition, Table 2 also presents the elemental composition and concentration levels of SSW. The very low oxides content of SSW speculates their inert nature, which favors their incorporation into clayey bodies to form stable products [49,51]. Most of all, the low oxides levels of SSW ensures the synergistic replacements of high proportions of clay materials with SSW without significant changes to the chemical constituents of the formed bricks. The dominance of K2O (2.11 wt.%) in SSW, which is typical of an organic material [52], shows the significant contribution of SSW to the inorganic fluxing content of the brick mixture. Thus, the chemical analysis of the pristine raw materials support the replacement of clay materials with SSW for structural construction application.
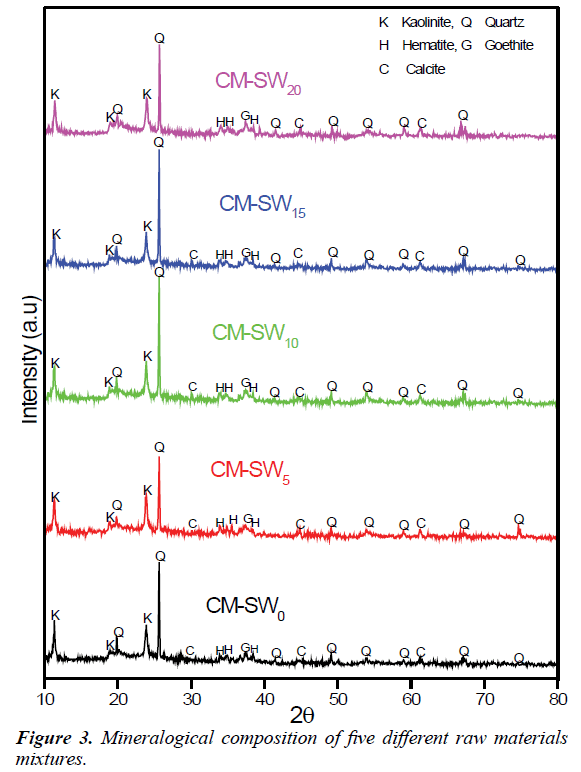
To validate the chemical composition analysis of the brick raw material, the X-ray diffractions analysis of the all brick mixtures were performed. Figure 3 shows the mineralogical compositions of the raw material mixtures. Perusing Figure 3 clearly shows similarities in diffraction patterns among the brick mixtures indicating the presence of same mineralogical composition. Analysis of the diffraction patterns commonly reveals the presence of quartz (SiO2), kaolinite (Al2Si2O5(OH)4), calcite (CaCO3), hematite (Fe2O3) and goethite (Fe(OH)). The intense quartz and kaolinite peaks semi-quantitatively relate to the dominance of SiO2 and Al2O3 content. The converse is also true for the case of CaCO3 and Fe2O3. The observed similarities in diffraction patterns imply SSW is most probably amorphous and inert materials. Thus, increasing replacement of clay material with SSW has no practical influence on the mineralogical content of the formed bricks. This analysis suggests significant amounts of SSW materials could be utilize in replacing clay materials for sustainable clay bricks construction. Thus, the mineralogical analysis of the raw materials is in agreement with their chemical compositions.
Further, Table 3 displays the results of the ultimate analysis test of SSW. The low ash content of SSW, which indicates its excellent organic composition, authenticates its minute inorganic oxide compositions (Table 2). Estimating the biofuel potential of SSW using Canseco-Medel equation (equation [1]) yielded a Higher Heating Value (HHV) of 3,283.06 kcal/kg [53].
| HHVa | C | H | N | S | Ob | Moisture | Ash |
|---|---|---|---|---|---|---|---|
| 3,283.06 | 47.95 | 5.83 | 3.17 | *ND | 41.70 | 0.29 | 4.52 |
Table 3. Spent shea waste organic composition expressed in wt.% and its biofuel value estimated as higher heating value (HHV) in kcal/kg.
HHV(kcal / kg) = 84[C]+ 227.65[H]+ 26.5[O]+ 25[S]+15[N] [1]
[O] =100 −[C] −[H] −[ash] [2]
Where [S] = O.
The non-detection of S in SSW as shown in Table 3 proposes the potential contribution of 3,283.06 kcal/kg heating energy from SSW is environmentally safe. The obtained biofuel value of SSW is within the range of reported values observed in literature on the analysis of other organic materials [43,53].
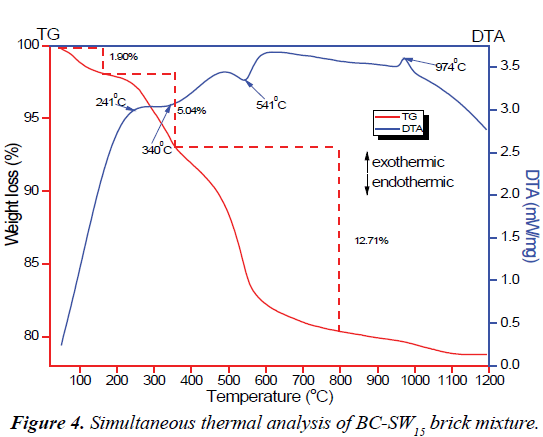
Tailoring the technological properties of bricks into desired forms mandate thorough comprehension of the thermal behavior of the raw materials at higher firing temperatures. Figure 4 illustrates the TG-DTA curves of CM-SW15 brick mixture as representative of most of the brick mixtures. The TG curve shows a three-step weight loss. The first 1.90 wt.% weight loss could be related to evaporation of moisture from the brick mixture. The second 5.04 wt.% corresponding to an exothermic peak occurring around 241°C indicate the decomposition of cellulose and proteins in SSW and other organic content present in clay materials. The final 12.74 wt.% weight loss relating to the endothermic peak at 541°C marks the transformation of kaolinite into metakaolinite via dehydroxylation reactions [54,55] and the degradation of hemicellulose, triacylglycerols and lignin components in SSW [25,29]. The broad endothermic peak at 340°C shows the dehydroxylation of goethite into hematite [56] whereas the sharp exothermic peak at 974°C indicate the structural transformation of metakaolinite into mullites [57]. Thus, the TG-DTA studies of the brick mixture are in agreement with its XRD results of the brick mixture.
Characterization of bricks technological properties
Physical-mechanical properties: The technological properties of the dried and fired brick specimens including green density, linear shrinkage and fired density of the prepared brick products are summarized in Table 4. The green densities of Series 1, 2, 3 and 4 reference standard dried brick specimens were 1.75 g/ cm3, 1.69 g/cm3, 1.72 g/cm3 and 1.76 g/cm3, respectively. The slight variation in green densities among the reference brick specimens is typical of a manual pressing method of shaping brick units. The progressive replacement of clay materials with high proportions of SSW showed a decreasing trend in green densities among the various brick series. The observed decreasing trend in green densities demonstrates the lightening effect of SSW on the bricks green weights, which intimates their relative low specific gravity nature. Earlier researchers have reported similar lightening phenomena on the replacement of clay material with organic content [58,59]. This analysis postulates the development of lightweight bricks from the replacement of clay materials with low specific gravity SSW.
| Firing temperature | Brick bars | Green density (g/cm3) | Fired density (g/cm3) | Fired shrinkage (%) |
|---|---|---|---|---|
| Series 1 (900 °C) |
CM-SW0 | 1.66 | 1.60 | 2.04 |
| CM-SW5 | 1.62 | 1.47 | 2.46 | |
| CM-SW10 | 1.52 | 1.32 | 3.09 | |
| CM-SW15 | 1.49 | 1.30 | 3.12 | |
| CM-SW20 | 1.44 | 1.22 | 3.56 | |
| Series 2 (1000 °C) |
CM-SW0 | 1.65 | 1.81 | 5.15 |
| CM-SW5 | 1.56 | 1.71 | 6.43 | |
| CM-SW10 | 1.52 | 1.72 | 6.97 | |
| CM-SW15 | 1.50 | 1.56 | 7.41 | |
| CM-SW20 | 1.44 | 1.49 | 7.66 | |
| Series 3 (1100 °C) |
CM-SW0 | 1.65 | 1.88 | 5.11 |
| CM-SW5 | 1.63 | 1.68 | 6.58 | |
| CM-SW10 | 1.48 | 1.73 | 7.12 | |
| CM-SW15 | 1.45 | 1.56 | 7.48 | |
| CM-SW20 | 1.45 | 1.44 | 7.78 | |
| Series 4 (1200 °C) | CM-SW0 | 1.66 | 2.02 | 4.99 |
| CM-SW5 | 1.58 | 1.79 | 6.36 | |
| CM-SW10 | 1.50 | 1.75 | 7.00 | |
| CM-SW15 | 1.45 | 1.65 | 7.32 | |
| CM-SW20 | 1.44 | 1.59 | 7.63 |
Table 4. Technological properties of prepared clay brick specimens.
Contrary to green density, the linear fired shrinkage of all the brick series increased at higher replacement of the clay material as well as increase in firing temperature. Linear fired shrinkage is a measure of the extent of contraction or expansion of bricks material as they are heated at elevated temperatures [38]. The bare increase in L.S% values among Series 1 brick specimens could be related to contractions dominated by decompositions of SSW. The marginal increase in L.S% in Series 2, 3 and 4 suggest contractions mainly governed by liquid phase formation of the clay material. As presented in Table 4, the L.S% values observed in this research is higher than those reported in cited literature [12,60]. Plausible reason for obtained results could be linked to the excellent kaolinite content of the clay material, which probably occasioned large contractions from dehydroxylation reactions. Nevertheless, in all brick Series, L.S% is found within 8% which ensure their good contraction quality [39,61].
In addition, the F.D values of reference standard brick specimens of Series 1, 2, 3 and 4 were 1.72 g/cm3, 2.07 g/cm3, 2.04 g/cm3 and 2.02 g/cm3, respectively as indicated in Table 4. F.D values of good quality bricks range between 1.8 g/cm3 and 2 g/cm3 [62]. The F.D value of 1.72 g/cm3 for Series 1 reference standard brick specimen, in this context could be regarded as low quality signifying firing at 900°C is not an optimized manufacturing condition. Similar to green density, the F.D values of all brick Series decreased at higher replacement proportions of clay material with SSW. The great relevance of this result shows that bricks fired above 900°C with F.D values below 1.8 g/cm3 (i.e CM-SW10-20) could be classified as good quality lightweight bricks. Lightweight bricks are indispensable to disaster zones (e.g. earthquake prone areas) structural construction [63]. The comparative consistent low F.D values of bricks fired at 900°C perhaps allude to limited vitrification of the clay materials. The close proximity of F.D values among the 1000°C and 1100°C fired brick specimens imply the occurrence of similar phase transformations at those temperatures.
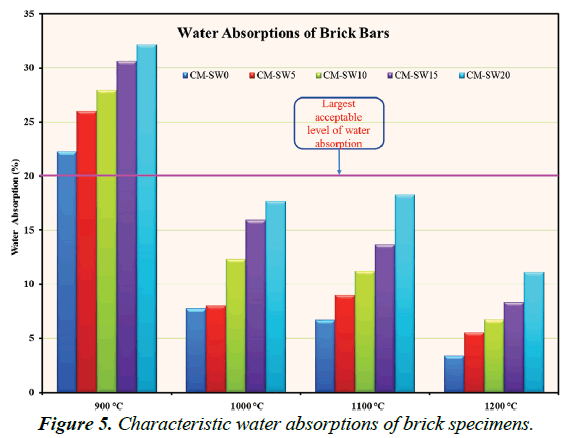
An indirect measure of open porosity, serviceability lifetime and hence an essential quality determinant parameter of fired brick products, are assessed based on their extent of water absorptions. Figure 5 portrays W.A% values of all brick specimens. As evident in Figure 5, the increase in W.A% value of the brick specimens formed from higher substitution of SSW for clay material relate to the increase in open porosity from the burn out of SSW within the matrices of those brick specimens. Within acceptable limits of quality standards requirements, pore creation in brick matrices increases the thermal insulation capacities of brick products thereby improving their energyconservation efficiencies. Excellent quality brick products exhibit W.A% values less than 20%; the lesser the better. In terms of W.A% values Series 1 (900°C) brick specimens were largely of low quality due to their high W.A% values. Series 4 (1200°C) brick specimens were of highest quality standards. Nevertheless, Series 2 (1000°C) brick specimens present most favorable manufacturing condition due to their good quality standards at economically low firing temperatures.
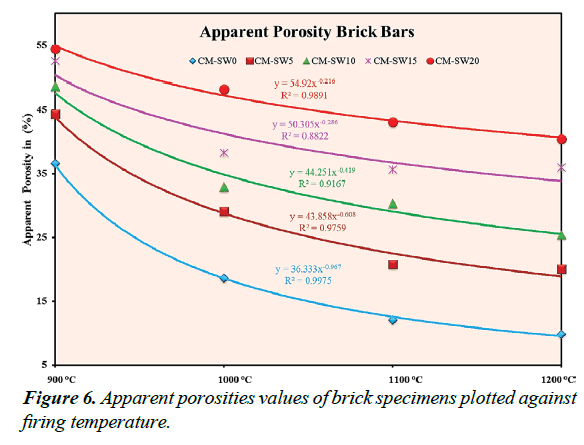
A measure of close pore circuitry of a brick system is expressed in terms of its A.P% value. Figure 6 shows the A.P% values of the brick specimens plotted as a function of increasing firing temperature. Evaluating Figure 6 shows that CM-SW5-20 brick specimens exhibit high A.P% values indicating their much porous nature relative to the reference standard brick specimens. This observation permits to hypothesis the improved thermal insulation properties of the CM-SW5-20 brick specimens. The decrease in A.P% values, at higher firing temperatures tentatively suggest the partial closure some pores most probably due to vitrification reactions. The extent of decrease was empirically found to follow obey a first order power series to a good extent.
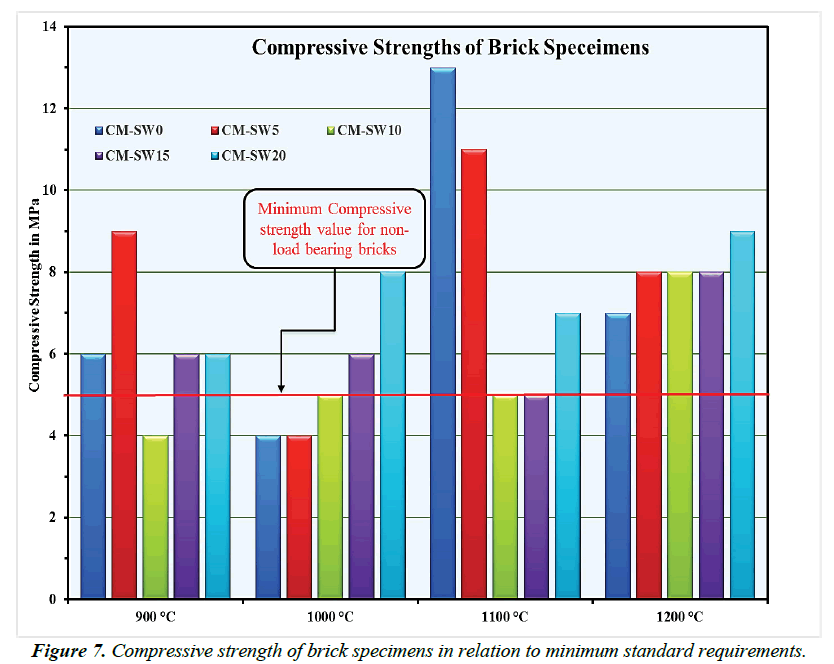
Nowadays the goal of achieving zero-energy building has placed emphasis on use of clay bricks with excellent thermal insulation properties for non-load bearing structural enclosures construction [18,64,65]. For this purpose, bricks must acquire the needed mechanical strengths just to self-support their own weights [18]. In this view, current building regulations set 5 Mpa as the minimum compressive strength value for non-load bearing structural construction bricks [66]. In addition, 2.5- 7.5 Mpa is the minimum approved range for use of horizontal perforated bricks in structural construction [67]. Besides, vertically perforated bricks mush attains minimum compressive strength values of 5 Mpa for use in structural construction purposes [68]. With this background, the compressive strengths of all brick specimens presented in Figure 7 largely met minimum standard requirements for use in structural construction. The apparent increase in compressive strength values at higher sintering temperatures could be related to the significant energy-contribution reactions of SSW [69,70]. Other researchers reported comparable range of compressive strengths on the substitution of clay materials for waste additives [38,71].
Morphological and thermal conductivity studies of brick specimens
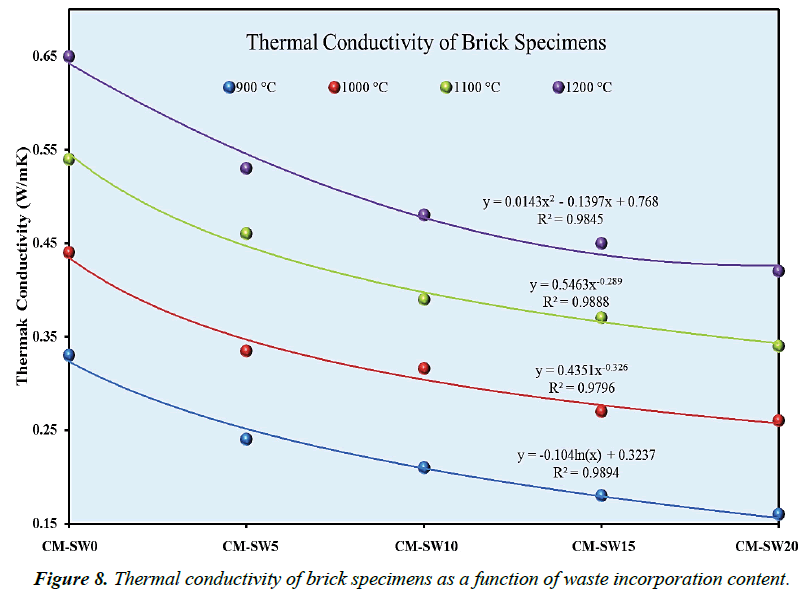
In addition to good mechanical resistance, use of bricks for energy-efficient building exceedingly depends on their thermal conductivities. Figure 8 gives a graphical illustration of thermal conductivities of the brick specimens plotted at various firing temperatures. As portrayed in Figure 8, the brick series generally revealed an increase in thermal conductivity as bricks were progressively fired at higher temperatures. However, the converse was not true in case of increasing clay replacement ratios with SSW. One great relevant of this analysis is that the thermal insulation properties of fired bricks could significantly be improved via the optimum proportionate replacement of clays with SSW as ecological construction material. It could therefore be inferred from Figure 7 that brick which high A.P% values due to their porous nature characteristically exhibit low thermal conductivity; whereas, bricks with low A.P% values have higher thermal conductivity. Thus, the nature of pores created within a brick matrix is a major controlling factor of thermal conductivity. In this view, the thermal conductivity of brick specimens are inversely related to their apparent porosities. Xu et al observed similar trend [38].
Figure 9A-9B shows the morphology of CM-SW15 brick specimens from SEM micrographs. The 900°C fired bricks showed morphology of fine network of numerous micropores due to burn out of SSW. Bricks sintered at 1000°C showed relative fewer networks of micropores but increased interconnectivity between the clay grains due to the formation of vitreous liquid phases. The 1100 °C fired bricks showed farther reduction in the micropores network due to the partial closure of these micropores from the dominant glassy phases. Finally, the 1200°C showed secondary porosity mainly due to the transformation of clay materials into plate-like mullites. According to Xu et al assertion, porous structure are extremely helpful in resisting convective heat transport better than completely vacant closed pores due to the reduction of free air circulation within the porous structures [38]. Thus, the thermal conductivity of porous bricks is much reduced compared to vacant enclosures. Our results are therefore in agreement with Xu et al [38].
Conclusion
Use of spent shea waste as renewable ecological construction material for sustainable production of construction clay bricks has been extensively examined in relation to their elemental, mineralogical, thermal, technological as well as morphological characteristics. The high content of Al2O3 and SiO2, low amount of Fe2O3 and CaCO3, good variety of inorganic oxides such as K2O, NiO, MnO, MgO, ZnO, Cr2O3, TiO2 and V2O5, and the excellent diversity of spent shea waste inorganic oxides mostly found in traces showed the suitability of the brick raw materials for use in structural construction works. The mineralogical characterization of the raw materials support the substitution of significant amounts of clay material for spent shea waste in forming brick units because of their inert nature. Thermal analysis of the raw material indicated the preferential transformation of the clay material into beneficial polyphases and the potential of gaining about 3,283.06 kcal/kg environmentally safe heating energy from spent shea waste. Replacing clay materials with spent shea waste showed overall improved technological performance of brick specimens in terms of reduction in weights, increase in compressive strengths and better thermal insulation properties at acceptable structural enclosure buildings standard requirements. Morphological studies provided photographic clues on the preferential transformation of the clay materials, decomposition of spent shea waste and the nature of the pore structures within the improved performing brick matrices. Overall, convincing baseline data on use of spent shea waste as renewable ecological material for sustainable production of construction clay bricks has been provided.
Acknowledgements
We acknowledge the funds provided Indian Council for Cultural Relations (ICCR) under their African Scholarship Scheme (G0179) for this research. In addition, we sincerely thank the staff and technicians of “Centralized Instrumentation and Service Laboratory” Annamalai University, for data analysis. Further, we are most grateful to Dr. V. Ramaswamy, Professor and Head, Department of Annamalai University, Tamilnadu, India, for his logistical support in carrying out this research. Finally, we are indebted Mrs. Gladys Ba-endeng Adazabra for airlifting the spent shea waste from Ghana to India.
References
- Oti JE, Kinuthia JM, Bai J. Design thermal values for unfired clay bricks. Mater Design. 2010;31(1):104-12.
- Abi CBE. Effect of borogypsum on brick properties. Constr. Build. Mater. 2014;59:195-203.
- Lingling X, Wei G, Tao W, et al., Study on fired bricks with replacing clay by fly ash in high volume ratio. Constr. Build Mater. 2005;19(3):243-7.
- Chen Y, Zhang Y, Chen T, et al., Preparation of eco-friendly construction bricks from hematite tailings. Constr. Build. Mater. 2011;25(4):2107-11.
- Kuusk K, Kalamees T, Maivel M. Cost effectiveness of energy performance improvements in Estonian brick apartment buildings. Ener Build. 2014;77:313-22.
- Al-Hadhrami LM, Ahmad A. Assessment of thermal performance of different types of masonry bricks used in Saudi Arabia. Appl Thermal Eng. 2009;29(5-6):1123-30.
- Bernardi D, DeJong JT, Montoya BM, et al., Bio-bricks: Biologically cemented sandstone bricks. Constr. Build Mater. 2014;55:462-9.
- Chen C, Li Q, Shen L, et al., Feasibility of manufacturing geopolymer bricks using circulating fluidized bed combustion bottom ash. Environ Technol. 2012;33(10-12):1313-21.
- Ahmari S, Zhang L. Utilization of cement kiln dust (CKD) to enhance mine tailings-based geopolymer bricks. Constr. Build Mater. 2013;40:1002-11.
- Menezes RR, Ferreira H, Neves GA, et al., Use of granite sawing wastes in the production of ceramic bricks and tiles. J Eur Ceram Soc. 2005;25(7):1149-58.
- Nirmala G, Viruthagiri G. FT-IR characterization of articulated ceramic bricks with wastes from ceramic industries. Spectrochim. Acta A. 2014;126:129–134.
- Sutcu M, Akkurt S. The use of recycled paper processing residues in making porous brick with reduced thermal conductivity. Ceram Int. 2009;35:2625-31.
- Quijorna N, Coza A, Andresa A, et al., Recycling of Waelz slag and waste foundry sand in red clay bricks. Resour. Conser Recycl. 2012;65:1-10.
- Ahmari S, Zhang, L. Production of eco-friendly bricks from copper mine tailings through geopolymerization. Constr. Build. Mater. 2012;29:323–331.
- Kumar A, Kumar S. Development of paving blocks from synergistic use of red mud and fly ash using geopolymerization. Constr Build Mater. 2013;38:865-71.
- Raut SP, Ralegaonkar RV, Mandavgane SA. Development of sustainable construction material using industrial and agricultural solid waste: A review of waste-create bricks. Constr Build Mater. 2011;25:4037-42.
- Mucahit S, Sedat, A. The use of recycled paper processing residue in making porous brick with reduced thermal conductivity. Ceram Int. 2009;35:2625-31.
- Muñoz Velasco P, Morales Ortíz M.P, Mendívil Giró MA, et al., Fired clay bricks manufactured by adding wastes as sustainable construction material – a review. Constr Build Mater. 2014;63:97-107.
- Bories C, Borredon M.-E, Vedrenne E, et al., Development of eco-friendly porous fired clay bricks using pore-forming agents: A review. J Environ Manag. 2014;143:186-96.
- Muñoz P, Morales MP, Mendívil M, et al., Using of waste pomace from winery industry to improve thermal insulation of fired clay bricks. Eco-friendly way of building construction. Constr Build Mater. 2014;71:181-7.
- Lamien N, Boussim JI, Nygard R, et al., Mistletoe impact on Shea tree (Vitellaria paradoxa C.F. Gaertn.) flowering and fruiting behaviour in savanna area from Burkina Faso. Environment and Experimental Botany. 2006;55:142-8.
- Bail S, Krist S, Masters E, et al., Volatile compounds of shea butter samples made under different production conditions in western, central and eastern Africa. J Food Comp Anal. 2009;22:738-44.
- Zhang J, Kurita M, Shinozaki T, et al., Triterpene glycosides and other polar constituents of shea (Vitellaria paradoxa) kernels and their bioactivities. Phytochem. 2014;108:157-70.
- Naughton CC, Lovett PN, Mihelcic JR. Land suitability modeling of shea (Vitellaria paradoxa) distribution across sub-Saharan Africa. Appl. Geo. 2015;58:217-27.
- Adazabra AN, Viruthagiri G, Ravisankar R. Cleaner production in the Shea industry via the recovery of Spent Shea Waste for reuse in the construction sector. J Clean Prod. 2016;122:335-44.
- Woods J, Tipper R, Brown G, et al., Evaluating the Sustainability of Co-firing in UK. Report No. URN 06/1960. Themba Technology Ltd, London, United Kingdom. 2006.
- Nimmo W, Daood SS, Gibbs BM. The effect of O2 enrichment on NOx formation in biomass co-fired pulverised coal combustion. Fuel. 2010;89:2945-52.
- Pousga S, Boly H, Lindberg JE, et al., Evaluation of traditional sorghum (Sorghum bicolor) beer residue, Shea-nut (Vitellaria paradoxa) cake and cotton seed (Gossypium spp) cake for poultry in Burkina Faso: availability and amino acid digestibility. Int J Poult Sci. 2007;6:666-72.
- Darvell LI, Jones JM, Gudka B, et al., Combustion properties of some power station biomass fuels. Fuel. 2010;89:2881-90.
- ASTM D-2974. Standard test method for moisture, ash, and organic matter of peat and other organic soils. 1987.
- EN 772-16. Methods of Test for Masonry Units – Part 16: Determination of Dimensions. 2011.
- State General Administration of the People's Republic of China for Quality Supervision and Inspection and Quarantine, GB/T2542-2003, Test Methods for Wall Bricks, Standards Press of China, Beijing, 2003.
- UNE-EN 772-1. Methods of test for masonry units – part 1: determination of compressive strength. 2002.
- Karaman S, Esmeray A. Determining the Conformity to standards of clay deposits in Tokat-Zile region as raw material in brick–tile production. KSU J Sci Eng. 2006; 9(1): 130-4.
- Tudisca V, Casieri C, Demma F, et al., Firing technique characterization of black-slipped pottery in Prasnesete by low field 2D NMR relaxometry. J Archaeol Sci. 2011;38:352-9.
- Nirmala G, Viruthagiri G. A view of microstructure with technological behavior of waste incorporated ceramic bricks. Spectrochim Acta A. 2015;135:76–80.
- Eliche-Quesada D, Iglesias-Godino FJ, Pérez-Villarejo L, et al., Replacement of the mixing fresh water by wastewater olive oil extraction in the extrusion of ceramic bricks. Constr Build Mater. 2014;68:659–66.
- Xu Y, Yann C, Xu B, et al., The use of urban river sediments as a primary raw material in the production of highly insulating brick. Ceram Int. 2014;40:8833-40.
- Yang C, Cui C, Qin J, et al., Characteristics of the fired bricks with low-silicon iron tailings. Constr Build Mater. 2014;70:36-42.
- Alonso-Santurde R, Andrés A, Viguri JR, et al., Technological behaviour and recycling potential of spent foundry sands in clay bricks. J Env Manag. 2011;92:994-1002.
- Bondar D, Lynsdale CJ, Milestone NB, et al., Effect of type, form and dosage of activators on strength of alkali-activated natural pozzolans. Cem. Conc. Comp. 2011;33 (2): 251-60.
- Ferone C, Liguori B, Capasso I, et al., Thermally treated clay sediments as geopolymer source material. Appl Clay Sci. 2015;107:195-204.
- Velasco PM, Ortiz MPM, Giró MAM, et al., Development of sustainable fired clay bricks by adding kindling from vine shoot: Study of thermal and mechanical properties. Appl Clay Sci.2015;107:156-64.
- García-Ten J, Silva G, Cantavella V, et al., Use of lightening materials in the manufacture of thermoarcilla block. Influence on the thermal conductivity and the behavior of the process. Conarquitectura. 2005;16:65-72.
- Velasco PM, Morales Ortíz M P, Mendívil Giró M, et al., Fired clay bricks manufactured by adding wastes as sustainable construction material - A review. Constr Build Mater. 2014;63;97-107.
- Bernhardt M, Justnes H, Tellesbo H, et al., The effect of additives on the properties of lightweight aggregates produced from clay. Cem. Conc Compo. 2014;53:233-8.
- Sultana MS, Ahmed AN, Zaman MN, et al., Utilization of hard rock dust with red clay to produce roof tiles. J Asian Ceram Soc. 2015;3:22-6.
- Karaman S, Gunal H, Ersahin S. Assessment of clay bricks compressive strength using quantitative values of colour components. Constr Build Mater. 2006;20:348-54.
- Faria KCP, Gurgel RF, Holanda JNF. Recycling of sugarcane bagasse ash waste in the production of clay bricks. J Environ Manag. 2012;101:7-12.
- Sena da Fonseca B, Galhano C, Seixas D. Technical feasibility of reusing coal combustion by-products from a thermoelectric power plant in the manufacture of fired clay bricks. Appl Clay Sci. 2015;104:189-95.
- Andrés A, Carmen Díaz M, Alberto Coz José AM, et al., Physico-chemical characterisation of bricks all through the manufacture process in relation to efflorescence salts. J Eur Ceram Soc. 2009;29:1869-77.
- Datta J, Chowdhury DP, Verma R, et al., Determination of elemental concentrations in environmental plant samples by instrumental neutron activation analysis. J. Radioanal Nucl Chem. 2012;294:261-5.
- Canseco-Medel A. Tecnología de Combustibles. Ed. Fundación Gómez-Pardo 2v, Madrid. 1978.
- Parthasarathy P, Sheeba NK, Arockiam L. Study on kinetic parameters of